RESEARCH ARTICLE
Volltext
Abbildung

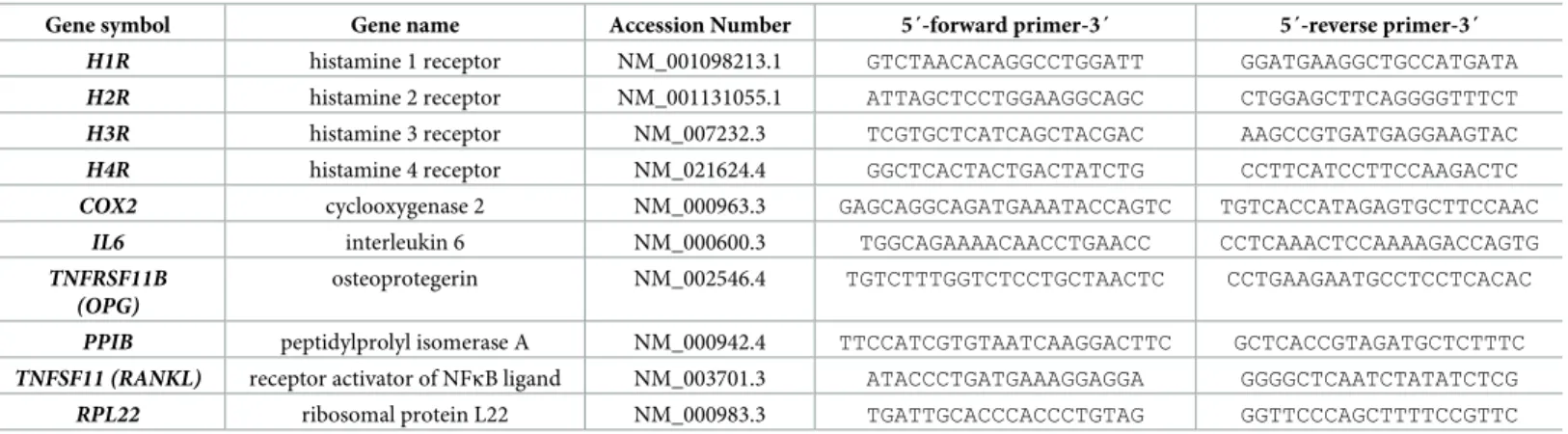
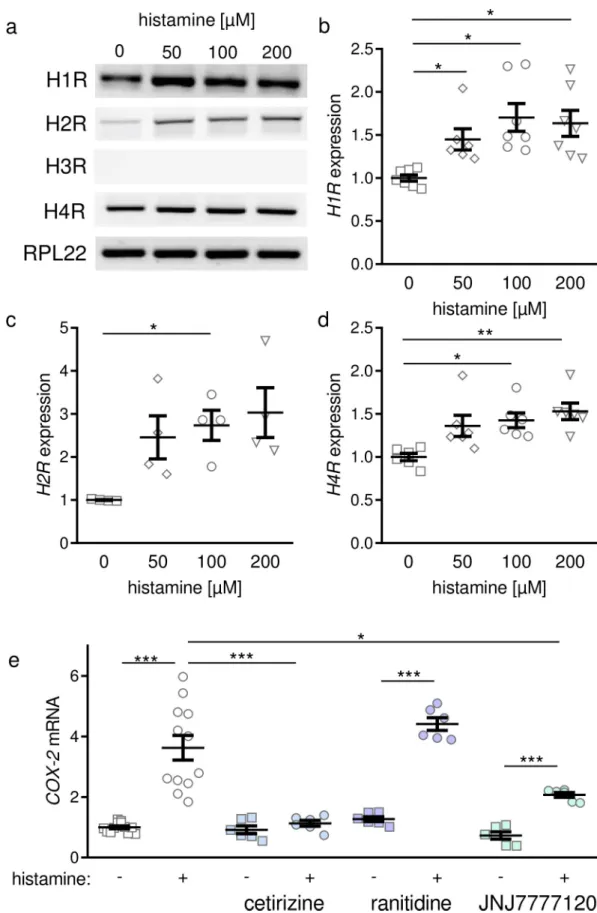
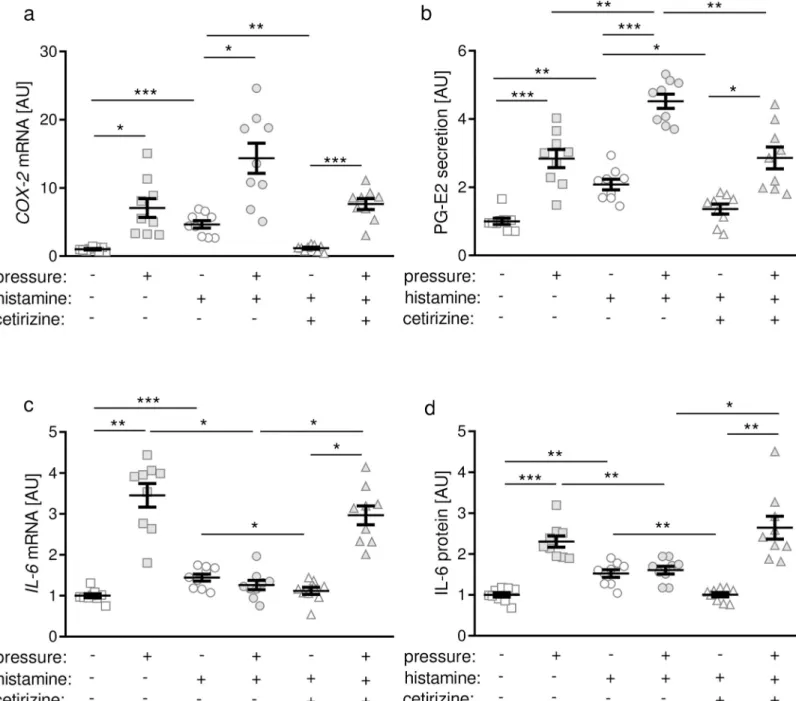
ÄHNLICHE DOKUMENTE
Despite much higher coccolithophore abundance in the greenhouse treatment, particulate inorganic carbon production (calcification) was significantly decreased by the combination
Eine Auswertung aller Metaanalysen zur therapeutischen Hypothermie durch die Brain Trauma Foundation hat zeigen können, dass eine therapeutische Hypo - thermie zwar nicht
(dee) MCF-7 and 2 different shLAMP2A cell lines were cultured in ovo, followed by staining and scoring of the tumor cells for LAMP2A (upper panel) and Ki-67 (lower panel)
Increased valvular expression of NF-κB in diabetic individuals is associated not only with serum HbA 1c and fructosamine levels but also with AVA and transvalvular gradient,
To evaluate patient clinical conditions and the effect of landiolol hydrochloride treatment on contrast media-induced anaphylactic shock treatment response, we reviewed the
However, compounds reported to release histamine from cells and thereby leading to arrhythmias in humans, namely morphine, ketamine, and fentanyl, failed to induce a more
The speeifity of the method was proved by thinlayer chromatography, degradation b y diamine oxidase (pig kidney) and histamine methyltransferase (guinea-pig brain),
Summary. High histamine concentrations and histamine methyl transferase activity were demonstrated in the gastric mueosa of man, dog, pig and cow. Modified methods for