Dermal fillers for tissue augmentation: an overview
Injizierbare Füllmaterialien zur Gewebeaugmentation: eine Übersicht
Abstract
Treatments with dermal fillers for tissue augmentation constitute the majority of all non-surgical procedures in plastic surgery. Newly de-
Philip H. Zeplin
1Ziah Taufig
2veloped products get launched and the market grows continuously, but
Peter M. Vogt
3the “ideal” substance has yet not been found. The substances used
Stefan Langer
1these days are high molecular compounds. They have substantial dif- ferences in their physicochemical properties and are suspended in complex matrices. This overview describes the latest history of dermal
1 Universitätsklinikum Leipzig, Department für Operative fillers and the commonly used substances of different origin and formal-
izes the need for the development of systematic procedures of stand-
Medizin, Abteilung für ardized pre-clinical tests with subsequent certification as well as the Plastische, Ästhetische und establishment of interdisciplinary clinical guidelines to ensure cus-
tumer’s safety.
spezielle Handchirurgie, Leipzig, Germany
Zusammenfassung
Die Behandlung mit injizierbaren Füllsubstanzen macht inzwischen einen Großteil aller nichtchirurgischen Eingriffe in der plastischen
2 Praxisklinik für Plastische Chirurgie, Köln, Germany 3 Medizinischen Hochschule
Hannover, Klinik für Plastische, Hand- und Chirurgie aus. Andauernd kommen neue synthetische Substanzen zur
Wiederherstellungschirurgie, Hannover, Germany Vermarktung, aber die Suche nach dem „idealen“ Füllstoff scheint noch
nicht abgeschlossen zu sein. Bei den heute verwendeten Materialien handelt es sich um hochmolekulare Verbindungen, die deutliche Unter- schiede in ihrem physikochemischen Verhalten aufweisen und in einer komplexen Matrix eingebunden sind. Diese Arbeit vermittelt eine Übersicht über die jüngste Geschichte injizierbarer Füllsubstanzen und gibt einen Überblick über die gebräuchlichen Substanzen unterschied- lichster Herkunft. Gleichzeitig wird die Notwendigkeit für die Entwicklung standardisierter präklinischer Tests zur Zertifizierung und die Entwick- lung einer interdisziplinären Leitlinie zur Gewährleistung der Patienten- sicherheit formuliert.
Introduction
With over 2 million treatments per year, dermal fillers for tissue augmentation make up a quarter of all non-surgical procedures in plastic surgery [1]. They may be used to correct single deep furrows, multiple fine lines or de- pressed scars. Filling materials are applied by injecting them underneath and within tissue areas using thin cannulas to create a smoother, firmer appearance and to replace volume loss. Depending on the type of filler material being used, they are expected to last several months or even years. Suitable injection sites are the nasolabial fold, perioral and mento-labial folds and the glabella fold. Newly developed products get launched and the market of synthetic substances and various compositions grows continuously. This shows that the
“ideal” substance has yet not been found. The substances used these days are high molecular compounds. They have substantial differences in their physicochemical properties and are suspended in a complex matrix. They
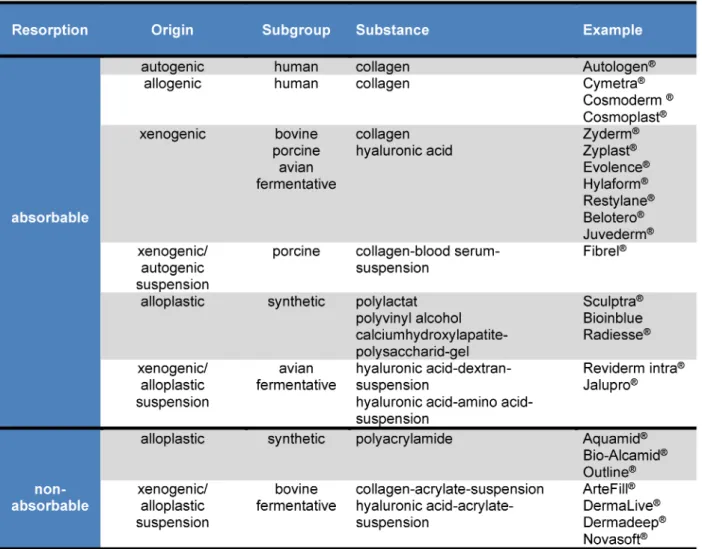
can be categorized into two groups – absorbable and non-absorbable materials, differing from each other in origin and production (Table 1).
Dermal fillers based on collagen
Collagens are fibrous extra-cellular proteins and make up the main component of soft tissue. Fillers based on collagen are used mainly for facial wrinkle reduction and for augmentation of the lips, but they can also be used for the enhancement of facial contour. At first, fillers based on collagen were made of bovine collagen (e.g.
Zyderm®, Zyplast®). The high standard of manufacturing procedures combined with the isolation of animals prac- tically excluded the risk of a contamination with BSE. Al- lergies towards bovine collagen made hypersensitivity testing prior treatment a requirement. With time, this lead to the gradual replacement of bovine collagen by the launch of human and porcine collagen products. The
1/4 GMS German Plastic, Reconstructive and Aesthetic Surgery 2014, Vol. 4, ISSN 2193-7052
Review Article
OPEN ACCESS
Table 1: Dermal fillers
treatment with autologous human collagen (e.g. Autolo- gen®) is a two-step process. At first, skin tissue is obtained surgically from a patient. Following that, collagen (mainly type I) gets extracted, sterilized and transferred into an injectable collagen suspension. This takes 3 to 4 weeks [2]. Allogenic human collagen is either obtained from skin fibroblast cultures (Cosmoderm®, Cosmoplast®) or micro- particles of decellularized dermis (Cymetra®). Autologous and allogenic human collagens are not the only sub- stances that do not require pre-treatment skin testing;
fillers based on collagen derived from porcine skin (Evolence®) have an equally high biocompatibility. In 1985, an alternative to the pre-existing collagens was developed which is particularly interesting for treating scars. Autologous-xenogeic gelatine matrix (Fibrel®) combines porcine collagen with patient blood. Preceding allergy testing is necessary but successful outcomes last 3–12 months [3].
Dermal fillers based on the
glycosaminoglycan hyaluronic acid
Hyaluronic acid [(C14H21NO11)n], a glycosaminoglycan, is one of the main components of the extracellular matrix
of the dermis in addition to other proteoglycans. It may be used without previous hypersensitivity testing. Hyalur- onic acid has hydrophilic properties, therefore it can sta- bilize the connective tissue by binding water molecules at the injection site. They are a popular choice for enhan- cing facial contour, enlargement of certain areas of the body, reducing wrinkles by filling in lines as well as cor- recting imperfections of the face. The substance was originally derived from chemically modified avian hyalur- onic acid (e.g. Hylaform®). Nowadays it is genetically en- gineered using bacterial fermentation (e.g.Streptococcus equi). The products differ from each other in the way the hyaluronic acid molecules are cross-linked with each other. Un-cross-linked hyaluronic acid is composed of molecules in their natural length. By adding cross-linking agents (e.g. Butandioldiglycidylether/BDDE) it is possible to elongate the natural chain of molecules, creating a meshwork and cross-links between the hyaluronic acid molecules. On top of that, cross-linking also stabilizes the molecules and delays their breakdown, prolonging the water binding properties. Biphasic products are mixtures of cross-linked and non-cross-linked hyaluronic acids, (e.g. Restylane®). Their more viscous consistency leads to a greater and longer lasting volume restoration.
Monophasic products are products made of either cross-
2/4 GMS German Plastic, Reconstructive and Aesthetic Surgery 2014, Vol. 4, ISSN 2193-7052
Zeplin et al.: Dermal fillers for tissue augmentation: an overview...
linked or non-cross-linked hyaluronic acids (e.g. Belotero®, Juvederm®) and are supposed to be softer and able to spread more evenly within the tissue. However, since monophasic products induce a less effective tissue reac- tion compared to biphasic ones, they come along with a less extensive plumping up effect [4], [5], [6], [7]. By raising the amount of cross-links and increasing homo- geneity it is possible to create 3D-matrices, which are able to correct even deeper creases and lipatrophies.
Xenogenous-alloplastic combinations combine hyaluronic acid with dextran microparticles (e.g. Reviderm intra®) or undissolved amino-acids (e.g. Jalupro®). They were de- signed to either induce the formation of new connective tissue after the degradation of the hyaluronic acid or to stimulate reepithelialisation of the skin. However, due to lack of knowledge about possible long-term side effects the societies of plastic surgeons and dermatologists dis- courage the use of such products [8].
Synthetic fillers
Filling materials of neither bacterial nor animal origin are usually based on compositions that contain poly-L-lactic acid, polyvinyl alcohol or hydroxyl apatite. Crystalline poly- L-lactic acid is used in the form of lyophilisating implants made of polylactate-microparticles, mannitol and carboxymethyl cellulose (e.g. Sculptra®). Treatments offer degradation at a controllable rate as well as having a stimulating effect on collagen synthesis. According to clinical studies, the results may last up to 18 months if the dermal filler is applied gradually over 2–3 treatments scheduled 4–6 weeks apart [9]. Polyvinyl alcohol [C2H4O]
is a synthetic thermoplastic polymer filling material in the form of a hydrogel that is supposed to create a long lasting temporary effect of up to 1.5 years (Bioinblue®).
Small-particle calcium hydroxyapatite [Ca5(OH)(PO4)3] suspended in carboxymethyl cellulose as a gel carrier (e.g. Radiesse®) are supposed to show good effects on deep naso-labial crease and on marionette lines on the chin due to their long-lasting stimulating effect on collagen synthesis [10], [11]. Moreover, they are used in particular for HIV-associated lipoatrophy [12], [13].
Permanent fillers
Permanent fillers are synthetic materials, synthetic-colla- gen-mixtures or synthetic-hyaluronic acid mixtures. They create a permanent augmentation effect by a homogen- ous tissue reaction. The most commonly used substance is polyacrylamide, the product of a polymerisation reaction of acrylamide and N,N-Methylbisacrylamidmonomeres.
Suspended in an aqueous gel, polyacrylamide (e.g.
Aquamid®, Bio-Alcamid®, Outline®) has a long half-life and is used mainly for augmentation of the nasolabial fold and the glabellar fold, the corners of the mouth and for the correction of facial defects on cheeks, chin and nose.
Most of the permanent xenogenous-alloplastic combina-
tions are based on acrylates such as polymethylmeth acrylate/PMMA [(C5H8O2)n], hydroxymethylacrylate (HEMA), ethylmethacrylate (EMA) or the co-polymer made of hy- droxymethylacrylate und ethylmethacrylate (pHEMA-EMA).
Permanent fillers contain non-absorbable PMMA-micro- spheres and are suspended in bovine collagen (e.g.
Artefill®). After the bovine collagen is absorbed, the micro- spheres promote the body to synthetize its own collagen.
Mixtures of hydrophilic pHEMA-EMA and cross-linked hy- aluronic acid (e.g. Dermalive®, Dermadeep®, Novasoft®) have similar effects. The particle size ranging from 40–110 µm is able to interfere with migration and degrad- ation and at the same time encourages the attachment of fibroblasts. The treatment of age-related lines and wrinkles with permanent and semi-permanent dermal fillers seems to be more efficacious compared with tem- porary fillers. The current evidence originating from cases series suggests that these fillers achieve their objective and appear at least as safe as temporary fillers in short term studies. But because long-term studies are rare standard organizations of plastic surgeons and dermato- logists solely recommend the use of resorbable sub- stances [14], [15]. However, taking everything into con- sideration, permanent fillers do have a higher risk of un- wanted, foreign-body associated reactions [11]. That is why standard organisations of plastic surgeons and der- matologists solely recommend the use of resorbable substances [14], [15].
Conclusion
Experience has shown that the large number of both po- tential (e.g. aesthetic) and necessary (e.g. reconstructive) treatments and the vast range of applications cannot be covered by a single ideal filling material. It will remain inevitable that with every patient the indication together with the advantages and disadvantages of the treatment need to be carefully evaluated before deciding on a filler material. Although permanent fillers have been used for decades, the long term effects and consequences of biological degradation are mostly unknown. As a result of clinical experience and retrospective studies they are more carefully watched these days. To ensure costumer safety beyond a level of well-founded research, it would be desirable to develop systematic procedures of stand- ardized pre-clinical tests with subsequent certification.
The establishment of interdisciplinary clinical guidelines in combination with a central database for documentation of results and side effects with an additional introduction of a patient card would further increase customer safety.
Notes
Competing interests
The authors declare that they have no competing in- terests.
3/4 GMS German Plastic, Reconstructive and Aesthetic Surgery 2014, Vol. 4, ISSN 2193-7052
Zeplin et al.: Dermal fillers for tissue augmentation: an overview...
References
1. ISAPS International Survey on Aesthetic/Cosmetic Procedures Performed in 2011. Available from: http://www.isaps.org/Media/
Default/global-statistics/ISAPS-Results-Procedures-2011.pdf 2. Sclafani AP, Romo T 3rd,Parker A, McCormick SA, Cocker R,
Jacono A. Autologous collagen dispersion (Autologen) as a dermal filler: clinical observations and histologic findings. Arch Facial Plast Surg. 2000 Jan-Mar;2(1):48-52.
3. Millikan L, Banks K, Purkait B, Chungi V. A 5-year safety and efficacy evaluation with fibrel in the correction of cutaneous scars following one or two treatments. J Dermatol Surg Oncol.
1991 Mar;17(3):223-9. DOI: 10.1111/j.1524- 4725.1991.tb03635.x
4. Eccleston D, Murphy DK. Juvderm® Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-72. DOI:
10.2147/CCID.S35800
5. Goodman GJ, Bekhor P, Rich M, Rosen RH, Halstead MB, Rogers JD. A comparison of the efficacy, safety, and longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: a multicenter, prospective, randomized, controlled, single-blind, within-subject study. Clin Cosmet Investig Dermatol. 2011;4:197-205. DOI: 10.2147/CCID.S26055 6. Nast A, Reytan N, Hartmann V, Pathirana D, Bachmann F,
Erdmann R, Rzany B. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011 Jun;37(6):768-75. DOI:
10.1111/j.1524-4725.2011.01993.x
7. Prager W, Steinkraus V. A prospective, rater-blind, randomized comparison of the effectiveness and tolerability of Belotero®
Basic versus Restylane® for correction of nasolabial folds. Eur J Dermatol. 2010 Nov-Dec;20(6):748-52. DOI:
10.1684/ejd.2010.1085
8. Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003 Sep-Oct;27(5):354- 66; discussion 367. DOI: 10.1007/s00266-003-3022-1 9. Schierle CF, Casas LA. Nonsurgical rejuvenation of the aging face
with injectable poly-L-lactic acid for restoration of soft tissue volume. Aesthet Surg J. 2011 Jan;31(1):95-109. DOI:
10.1177/1090820X10391213
10. Alam M, Havey J, Pace N, Pongprutthipan M, Yoo S. Large-particle calcium hydroxylapatite injection for correction of facial wrinkles and depressions. J Am Acad Dermatol. 2011 Jul;65(1):92-6. DOI:
10.1016/j.jaad.2010.12.018
11. Comite SL, Liu JF, Balasubramanian S, Christian MA. Treatment of HIV-associated facial lipoatrophy with Radiance FN (Radiesse).
Dermatol Online J. 2004;10(2):2.
12. Silvers SL, Eviatar JA, Echavez MI, Pappas AL. Prospective, open- label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plast Reconstr Surg. 2006 Sep;118(3 Suppl):34S-45S.
DOI: 10.1097/01.prs.0000234847.36020.52
13. Sturm LP, Cooter RD, Mutimer KL, Graham JC, Maddern GJ. A systematic review of dermal fillers for age-related lines and wrinkles. ANZ J Surg. 2011 Jan;81(1-2):9-17. DOI:
10.1111/j.1445-2197.2010.05351.x
14. Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, Menezes J, Ferreira MC. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008 May;121(5):1811-20.
15. Ono S, Ogawa R, Hyakusoku H. Complications after
polyacrylamide hydrogel injection for soft-tissue augmentation.
Plast Reconstr Surg. 2010 Oct;126(4):1349-57. DOI:
10.1097/PRS.0b013e3181ead122
Corresponding author:
Dr. med. habil. Philip H. Zeplin
Universitätsklinikum Leipzig, Department für Operative Medizin, Abteilung für Plastische, Ästhetische und spezielle Handchirurgie, 04103 Leipzig, Germany, Phone:
+49-341-9717140
philip.zeplin@medizin.uni-leipzig.de
Please cite as
Zeplin PH, Taufig Z, Vogt PM, Langer S. Dermal fillers for tissue augmentation: an overview. GMS Ger Plast Reconstr Aesthet Surg.
2014;4:Doc06.
DOI: 10.3205/gpras000025, URN: urn:nbn:de:0183-gpras0000258
This article is freely available from
http://www.egms.de/en/journals/gpras/2014-4/gpras000025.shtml Published:2014-06-11
Copyright
©2014 Zeplin et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by-nc-nd/3.0/deed.en). You are free: to Share — to copy, distribute and transmit the work, provided the original author and source are credited.
4/4 GMS German Plastic, Reconstructive and Aesthetic Surgery 2014, Vol. 4, ISSN 2193-7052
Zeplin et al.: Dermal fillers for tissue augmentation: an overview...