Baudin et al.: Angiotensin I-converting enzyme assay in urine 857
J. Clin. Chem. Clin. Biochem.
Vol.28, 1990, pp. 857-861
© 1990 Walter de Gruyter & Co.
Berlin · New York
A Reliable Radiometrie Assay for the Determination of Angiotensin I-Converting Enzyme Activity in Urine
By B. Baudin, B. Beneteau-Burnat, F. Ch. Baumann*) and J. Giboudeau Laboratoire de Biochimie A, Höpital Saint-Antoine, Paris
Laboratoire de Chimie Biologique, UFR Pharmacie, Paris V, France
(Received November 28, 1989/June 11, 1990)
Summary: We present a radiometric assay for the determination of urinary angiotensin-converting enzyme activity, using benzoyl-[l-14C]glycyl-L-histidyl-L-leucine as the substrate. An optimal pH of 8.3, an optimal chloride concentration of 0.375 mol/1 and complete inhibition by EDTA-Na2, captopril and enalaprilat confirm the specificity of the assay. Comparison of dialysis and ultrafiltration for concentration of urine showed the existence of angiotensin-converting enzyme inhibitors in human urine. Dialysis against water was the more effective method for avoiding enzyme inhibition. After dialysis of urine, the assay was linear with time and with enzyme concentration; it was highly sensitive (60 mU/1) and showed good reproducibility. Under our technical conditions, we found angiotensin-converting enzyme activity in urine samples with quantitatively abnormal protein contents, but not in normal urine. Urinary angiotensin-converting enzyme did not correlate with proteinuria nor with water-salt parameters or creatinine. We confirm the kidney tubular epithelial origin of the enzyme, and propose the use of our assay to study urinary angiotensin-converting enzyme as a marker of renal tubular damage.
Introduction
Angiotensin I-converting enzyme is the peptidyldi- peptide hydrolase (dipeptidylcarboxypeptidase EC 3.4.15.1) which cleaves angiotensin I to the potent vasopressor angiotensin II and inactivates the vaso- dilator bradykinin. Angiotensin-converting enzyme activity is measurable in plasma and in most organs;
the enzyme is located on the luminal surface of the endothelial cells as an ecto-glycoprotein (1).
The brush border of kidney tubules is one of the extravascular sites richest in angiotensin-converting activity (2). Because of this location some authors have suggested the potential use of urinary angioten- sin-converting activity as an index of renal tubular damage (3, 4). Studies on urinary angiotensin-con- verting enzyme are, however, rare, possibly because its activity is low in urine and the accuracy of the
!) Deceased on September 1988
present assays not proved; in particular, no criteria of reliability have been specified (3 — 5). In addition, mammalian urine may contain angiotensin-convert- ing enzyme inhibitors (5).
In this study, we describe a sensitive and specific radiometric assay for determination of urinary angio- tensin-converting activity. We draw attention to the presence of inhibitors of the enzyme in human urine, and propose a prior dialysis of urine for an accurate determination of the enzyme. Concentration of urine before the measurement must be avoided.
Materials and Methods
Reagents
All reagents were of analytical grade. Potassium phosphates, sodium chloride and ethyl acetate were from Merck (Darm- stadt, FRG). EDTA-Na2 and 4-(2-hydroxyethyl)-l-piperazine- ethanesulphonic acid (HEPES) were provided by Sigma Chem- ical Co. (Saint Louis, Mo., USA). Benzoylglycyl-L-histidyl-L-
leucine was from Calbiochem (San Diego, CaL, USA) and benzoyl-[l-14C]glycyl-L-histidyl-L-leucine was purchased from New England Nuclear (Boston, Ma., USA). Instafluor was from Packard Instruments (Warrenville, 111., USA).
Captopril and enalaprilat were gifts from Squibb & Sons (Prin- ceton, NJ., USA) and Merck Sharp & Dohme (West Point, Pa., USA), respectively. PM-10 (cut-off Mr 10000) and XM-50 (cut- off Mr 50000) ultrafiltration membranes were from Amicon Ltd. (Lexington, Ma., USA), dialysis membranes from Union Carbide (Chicago, 111., USA) (cut-off Mr 1000).
Angiotensin-converting enzymatic assay
All urinary analyses were performed on 24-hour, fresh urine containing no preservatives, which was centrifuged 10 min at 2000 g, +4°C. Extensive dialysis and concentration by ultra- filtration were performed at 4- 4 °C with constant agitation on 10 ml aliquots of centrifuged urine.
The buffered substrate contained 0.165 mmol/1 radiolabelled substrate and 2.175 mmol/1 cold substrate in 0.5 mol/1 potas- sium phosphate — 0.75 mol/1 sodium chloride — pH = 8.3 buffer. Incubation was for one hour at 37 °C in 100 χ 8 mm glass tubes, started by the addition of 25 μΐ of urine to 25 μΐ of buffered substrate. The hydrolysis was stopped by the addition of 50 μΐ HC1 1 mol/1. Benzoyl-[l-14C]glycine was then extracted with 375 μΐ of ethyl acetate. After vigorous mixing and centrif- ugation (10 min at 1000g, +4°C), 250 μΐ of the upper ethyl acetate layer were directly dropped into 5 ml of Instafluor and counted (Mini-Maxi-Tricarb Packard — Packard Instruments).
Angiotensin-converting enzyme activity was determined in du- plicate and a blank was run with 0.15 mol/1 sodium chloride replacing the urine.
Results are expressed as angiotensin-converting enzyme units per litre of urine, one unit (U) corresponding to the release of one μηιοί of benzoylglycine in one minute at 37 °C.
The formula used is derived from that described in 1. c. (6) after calculation of the specific activity of the isotopic dilution (SA):
[Bq (dosage) - Bq (blank)]
' SA χ 0.91 χ 0.67 χ 60 χ 25 χ 10~6
_ ABq
~ SA χ 915 χ ΙΟ'6'
where 0.91 is the benzoylglycine fraction extracted by ethyl acetate; 0.67 is the counted organic fraction; 60 is time in minutes and 25 χ 10~6 the volume of the sample in litres.
For example, with radiolabelled substrate of activity 88000 Bq/μιηοΐ, SA becomes 7000 Bq/μιηοΐ, thus
Other determinations
Proteinuria, creatininuria, urinary sodium (Na) and potassium (K) were measured on the multiparametric discrete analysers Greiner G300 (Langenthal, CH) and Astra 8 (Beckman Instru- ments Inc. - Fullerton, Ca., USA).
Albumin/globulins ratio was determined with a Cliniscan den- sitometer (Helena Laboratories — Beaumont, Te., USA) after electrophoretic separation on agarose gel (Paragon SPE-II Kit, Beckman).
Urines
Normal urines were from 15 apparently healthy people from the laboratory staff (8 women, mean age 27.0 ± 10.8 years, and 7 men, mean age 30.1 ±10.7 years) without any renal
disorder as judged by a normal diuresis and normal urinary biological criteria: proteins < 0.15 g/1, Na = 131.1 ±49.0 mmol/1, K = 48.5 ± 15.8 mmol/1, creatinine = 12.2 ± 4.7 mmol/1.
Urines with an abnormally high protein content were selected from 44 urinary samples sent to the laboratory for electropho- retic analysis of quantitatively abnormal proteinuria (i.e.
>0.15g/day).
Data analysis
Results are expressed as mean ± standard deviation. Coeffi- cients of variation (CVs) for reproducibility studies were ob- tained from 20 determinations. Statistical comparisons were performed with the non-parametric Mann-Whitney U-test.
Results
As we did not find any angiotensin-converting activity in urines from normal subjects, we performed further experiments on urines with high protein contents. A direct determination showed the presence of angio- tensin-converting activity in 16 of the 44 urine samples tested. Concentration of these urines by ultrafiltration on Amicon PM-10 did not increase angiotensin-con- verting activity, but 24-hour dialysis against pure water increased the enzymatic activity by 85 ± 42%.
Dialysis against water was more effective than dialysis against the phosphate buffer of the enzymatic assay, or against 0.375 mol/1 sodium chloride, or a 25 mmol/1 HEPES - 0.375 mol/1 NaCl - pH = 8.3 buffer.
Concentration of the dialysates on Amicon PM-10 never increased the enzymic activity, and more often led to a substantial loss of activity. Dialysis against water did not reveal any angiotensin-converting ac- tivity in the other 28 urines with a high protein content or in the normal urines. For the experiments reported below, all urines were dialysed for 24 hours against pure water. Under these conditions we determined the main analytical parameters of the radiochemical as- say.
The specificity was assessed by
(i) an optimal pH of 8.3 (fig. 1 b) and activation by an increase in the chloride concentration (fig. 1 a), both of which are characteristic of angiotensin-con- verting enzyme activity determined with benzoylgly- cine-histidyl-leucine as the substrate;
(ii) a complete inhibition of urinary angiotensin-con- verting activity by 60 mmol/1 EDTA-Na2 (angioten- sin-converting enzyme is a zinc metallopeptidase) and 100 μηιοΐ/ΐ captopril or 40 μηιοΐ/l enalaprilat (two specific synthetic inhibitors of the enzyme). As phos- phates have been implicated as inhibitors of angio- tensin-converting activity, we verified that the con- centration used (250 mmol/1) was not inhibitory. In J. Clin. Chem. Clin. Biochem. / Vol. 28, 1990 / No. 11
Baudin et al.: Angiotensin I-converting enzyme assay in urine 859
^ ΟΛ
0.3
0.2 g 0.1
"
0.125 0.25 0.375 NaCI[mol/l]
0.5
Fig. 1. Urinary angiotensin-converting enzyme activity; pH and Chloride activation curves. Each point is the mean of three determinations of a pool of urinary samples dialysed against water.
particular, substitution of the phosphate buffer by a 50 mmol/1 HEPES - 0.75 mol/1 NaCl - pH = 8.3 buffer did not increase the urinary angiotensin-con- verting activity.
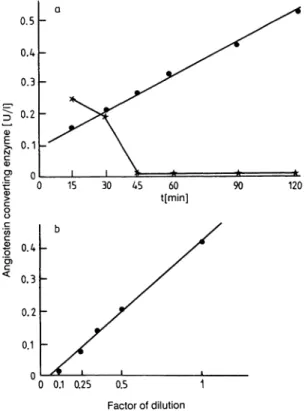
The assay was linear as a function of enzyme concen- tration up to 4.0 U/l and as a function of time up to 120 minutes (fig. 2). When the urines were not dia- lysed, the activity curves fell rapidly, suggesting an inhibitory agent in the urines which can be eliminated by dialysis (not shown). When, after dialysis, the urines were concentrated on PM-10, the activity curve swiftly decreased but not when a XM-50 membrane was used instead of the PM-10 membrane (fig. 2a).
These data suggest the existence of a second inhibitory substance in human urines eliminated neither by di- alysis nor by ultrafiltration on PM-10 membrane, but excluded by ultrafiltration on XM-50.
The sensitivity of the assay was determined by meas- uring the radioactivity of 30 blanks in the same set of experiments; the precision of the counts corre- sponded to an assay sensitivity of 44 mU/1. Linear dilution of urines, previously dialysed against water, showed the limit of detection to be 66 mU/1 (fig. 2b).
Within-day reproducibility CVs were less than 1% for two pools of urines with activities of 1.44 U/l and 3.21 U/l. Their between-days CVs were 10.3 and 4.4%
respectively. CVs were between 10 and 20% for a urine pool containing 124 mU/1 angiotensin-convert- ing activity.
In the 16 urines containing angiotensin-converting activity the mean activity was 2.6 ± 4.7 U/l with a range of 0.1 to 14.5 U/l and a median value of 440 mU/1, but the distribution of the values was not unimodal. There was no apparent difference between men and women. For these 16 urines, proteinuria was
0.5 ΟΛ 0.3 0.2 0.1
15 30 45 60
t[min] 90 120
0.4 0.3 0.2 0.1
0 0.1 0.25 0.5 1 Factor of dilution
Fig. 2. Radioassay of angiotensin-converting enzyme in urines, showing activity with time (a), and linearity (b). Each point is the mean of three determinations of a pool of urinary samples, dialysed only (·) or concentrated on Amicon PM-10 after dialysis (*).
6.7 ±15.1 g/1 (range: 0.35-62.4 g/1). Urinary angi- otensin-converting activity did not correlate with pro- teinuria (fig. 3), or with the albumin/globulins ratio (1.79 ± 1.06), or with any kind of abnormality on the agarose electrophoresis pattern, such as a peak in the γ-globulins or patterns of selective or non-selective proteinuria (not shown).
z> 13
σ)10
5 10Proteinuria [g/l] 60 Fig. 3. Lack of correlation (r = 0.29) between angiotensin-
converting activity and proteinuria measured in 16 urine samples with detectable enzyme activity.
Angiotensin-converting activity in urines was not re- lated to water-salt excretion; thus, no statistical link appeared between the enzyme activity and urinary Na (39.7 ± 35.1 mmol/1), K (34.0 ± 13.9 mmol/1), or Na/K (1.45 ± 1.49). Moreover, angiotensin-convert- ing activity was not linked to creatininuria (8.16
± 7.05 mmol/1). These data were the same when enzyme activity was expressed as U/g of protein or as U/mmol of creatinine.
Discussion
We modified the radiometric method of Rohrbach (6) for the determination of urinary angiotensin-convert- ing enzyme activity. Dilution of the radiolabelled sub- strate, benzoyl-[l -14C]glycyl-L-histidyl-L-leucine, with cold substrate gives a sensitivity suitable for a direct determination on urine samples, i.e. without prior concentration.
Dialysis of the urines is necessary to eliminate at least one inhibitor, which, as previously suspected (5), is of low molecular weight. According to the results ob- tained with PM-10 and XM-50 ultrafiltration mem- branes, another inhibitor may also be present; this inhibitor, relative molecular mass 10000 — 50000, is less inhibitory than the dialysable inhibitor since we could not reveal its inhibitory effect on non-concen- trated urines. Some ions and metals have been impli- cated as physiological angiotensin-converting enzyme inhibitors (5, 7); Lieberman described inhibition by human plasma but did not identify the agent respon- sible (8). Hazato & Kase found an inhibitor in pig plasma and identified it as an oligopeptide (9). Such low molecular weight inhibitors have been detected also in urine. Potential physiological inhibitors of higher molecular weight have also been reported, i. e.
albumin and some of its fragments (10), or fibrinogen fragments (11). In our study, it was difficult to cor- relate the inhibitory power of the urines with their protein concentration because we had only a few samples with both low angiotensin-converting activity and high protein content. Further investigations are necessary to characterize these urinary inhibitors of angiotensin-converting enzyme activity.
The reliability of our radiochemical enzyme assay after dialysis of urines is assessed by its specificity, linearity and reproducibility. The sensitivity is higher than that of most the methods described for the determination of angiotensin-converting activity in plasma. Only fluorimetric assays attain this sensitivity (12), but radioassays avoid the interferences fre- quently encountered with fluorimetric as well as pho- tometric methods (13). Kokubu et al. (14) adapted
Cushmarfs spectrophotometric assay (13) to urines but their method involves partial purification of the enzyme from the urine. Pitotti et al. (15) described a HPLC method but, once again, with difficult pretreat- ment of the urinary samples, and with no indication of the reliability or precision. A colorimetric assay was also proposed but without evidence of specificity for angiotensin-converting enzyme (3). Ryan et al. (5), like the present authors (personal data), were unable to adapt Cushman's assay for the determination of angiotensin-converting activity in mammalian urines, since the latter contain high concentrations of pig- ments. On the other hand, the radiochemical assay of Rohrbach (6) has poor sensitivity, detecting 500 mU/1, which is about 10-fold the limit of detection of our assay; thus Rohrbach's assay cannot be directly adapted to urine determination.
Under our technical conditions we could not detect angiotensin-converting activity in the urines of normal subjects, but we were able to measure the enzyme activity in urines with a quantitatively abnormal pro- tein content. Some authors (14,15) found angiotensin- converting activity in normal, 24-hour urines; possibly they were measuring the physiological replacement of the renal tubular brush-border whose epithelial cells are sedimentable. In view of the high specific angio- tensin-converting activities in the male genital tract (16, 17), a gonadic or prostatic origin of urinary angiotensin-converting enzyme might have been ex- pected, but we found no difference between males and females; in particular, normal men did not excrete angiotensin-converting activity in the urine. The ab- sence of a correlation between this urinary enzymatic activity and proteinuria, and even more the lack of dependence of this activity on the indices of glomer- ular function, such as creatininuria or albuminuria, eliminate the possibility of a passage of the plasmatic enzyme throughout the glomerular filter. Damage of renal glomeruli cannot explain a significant enzymu- ria, since glomerular epithelial and endothelial cells contain only a little angiotensin-converting enzyme (18, 19). Thus, in agreement with other authors (3, 4), we conclude that urinary angiotensin-converting enzyme is of tubular origin, as in the case of most enzymurias (20). We also showed that urinary angio- tensin-converting enzyme excretion is independent of sodium and potassium excretion, so that physiological or pathological variations of this enzyme in response to haemodynamic or dietary factors would not be expected.
Our specific, sensitive and reproducible radioassay might enable the comparison of angiotensin-convert- ing enzyme with other materials of tubular origin for their suitability as specific, early and precise markers
J. Clin. Chem. Clin. Biochem. / Vol. 28, 1990 / No. 11
Baudin et al.: Angiotensin I-converting enzyme assay in urine 861 of tubular damage during renal disorders. The assay
will also be of use in investigating putative inhibitors of angiotensin-converting enzyme in mammalian bi- ological fluids.
Acknowledgement
We wish to express our gratitude to Dr. Georges Morgant for statistical consultation and Yolande Tilly for typing the manu- script.
References
1. Caldwell, P. R. B., Seegal, B. C, Hsu, K. C, Das, M. &
Soffer, R. L. (1976) Angiotensin-converting enzyme vas- cular endothelial localization. Science 797, 1050—1051.
2. Oshima, G., Geese, A. & Erdös, E. G. (1974) Angiotensin I-converting enzyme of the kidney cortex. Biochim. Bio- phys. Acta 350, 26-37.
3. Baggio, B., Favaro, S., Cantaro, S., Bertazzo, L., Frunzio, A. & Borsatti, A. (1981) Increased urine angiotensin I- converting enzyme activity in patients with upper urinary tract infection. Clin. Chim. Acta 109, 211-218.
4. Kato, I., Takada, Y., Nishimura, K., Hiwada, K. & Ko- kubu, T. (1982) Increased urinary excretion of angiotensin- converting enzyme in patients with renal diseases. J. Clin.
Chem. Clin. Biochem. 20, 473-476.
5. Ryan, J. W., Martin, L. C., Chung, A. & Pena, G. A. (1979) Mammalian inhibitors of angiotensin-converting enzyme (kininase II). Adv. Exp. Med. Biol. 120 B, 599-606.
6. Rohrbach, M. S. (1978) [Glycine-l-14C]hippuryl-L-histidyl- L-leucine: a substrate for the radiochemical assay of angi- otensin-converting enzyme. Anal. Biochem. 84, 272 — 276.
7. Galardy, R. E. (1982) Inhibition of angiotensin-converting enzyme by phosphoramidates and polyphosphates. Bio- chemistry 27, 5777-5781.
8. Lieberman, J. & Sastre, A. (1986) An angiotensin-convert- ing enzyme (ACE) inhibitor in human serum. Increased sensitivity of the serum ACE assay for detecting active sarcoidosis. Chest 90, 869-873.
9. Hazato, T. & Käse, R. (1986) Isolation of angiotensin- converting enzyme inhibitor from porcine plasma.
Biochem. Biophys. Res. Commun. 739, 52 — 55.
10. Klauser, R. J., Robinson, C. J. G., Marinkovic, D. V. &
Erdös, E. G. (1979) Inhibition of human peptidyldipepti- dase (angiotensin I-converting enzyme: kininase II) by hu- man serum-albumin and its fragments. Hypertension 7, 281-286.
11. Saldeen, T., Ryan, J. W. & Berryer, P. (1981) A peptide derived from fibrinogen inhibits angiotensin-converting en- zyme and potentiates the effects of bradykinin. Thromb.
Res. 23, 465-470.
12. Friedland, J. & Silverstein, E. (1976) A sensitive fluorime- tric assay for serum angiotensin-converting enzyme. Am.
J. Clin. Pathol. 66, 416-424.
13. Cushman, D. W. & Cheung, H. S. (1971) Spectrophoto- metric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 20, 1637 — 1648.
14. Kokubu, T., Kato, L, Nishimura, K., Hiwada, K. & Ueda, E. (1978) Angiotensin I-converting enzyme in human urine.
Clin. Chim. Acta 89, 375-379.
15. Pitotti, A., Maurich, V., Moneghini, M. & Vianello, S.
(1986) HPLC method for evaluation of urinary angiotensin- converting enzyme: some examples of normal subjects and patients with renal transplantation. J. Pharmac. Biomed.
Anal. 4, 677-683.
16. Berg, T., Sulner, J., Lai, C. V. & Soffer, R. L. (1986) Immunohistochemical localization of two angiotensin I- converting isoenzymes in the reproductive tract of the male rabbit. J. Histochem. Cytochem. 34, 753-760.
17. Yokoyama, M., Hiwada, K., Kokubu, T., Takada, M. &
Takeuchi, M. (1980) Angiotensin-converting enzyme in hu- man prostate. Clin. Chim. Acta 700, 253-258.
18. Bruneval, P., Hinglais, N., Alhenc-Gelas, F.,'Tricottet, V., Corvol, P., Menard, J., Camillieri, J. P. & Barietti, J. (1986) Angiotensin I-converting enzyme in human intestine and kidney. Ultrastructural and immuno-histochemical locali- zation. Histochemistry 85, 73-80.
19. Chanse, L. D., Morin, J. P., Borghi, H., Ardaillou, N. &
Ardaillou, R. (1987) Angiotensin I-converting enzyme in isolated human glomeruli. FEBS Letters 220, 247-252.
20. Flandrois, C., Gravagna, B., Maire, I. & Mathieu, M.
(1986) Enzymurie. Ann. Biol. Clin. 44, 486-490.
Bruno Baudin Ph. D.
Laboratoire de Biochimie A Hopital Saint Antoine 184, rue du Fbg St-Antoine F-75571 Paris Cedex