Layer-by-layer nanoparticles for glaucoma therapy
Dissertation to obtain the Degree of Doctor of Natural Sciences (Dr. rer. nat.)
from the Faculty of Chemistry and Pharmacy University of Regensburg
Presented by Michaela Guter
from Kirchberg/Iller
February 2018
This work was carried out from January 2014 until December 2017 at the Department of Pharmaceutical Technology of the University of Regensburg.
The thesis was prepared under supervision of PD Dr. Miriam Breunig.
Submission of the PhD application: February 15, 2018
Date of examination: April 11, 2018
Examination board: Chairman: Prof. Dr. Jörg Heilmann
1st Expert: PD Dr. Miriam Breunig
2nd Expert: Prof. Dr. Rudolf Fuchshofer
3rd Examiner: Prof. Dr. Achim Göpferich
To my family
Table of Contents
Chapter 1 Introduction
Hyaluronan as a promising excipient for ocular drug delivery 9
Chapter 2 Goals of the Thesis 59
Chapter 3 Layer-by-layer assembled nanoparticles for siRNA delivery 73
Chapter 4 Hyaluronic acid decorated nanoparticles for the targeting of
trabecular meshwork cells 107
Chapter 5 Layer-by-layer nanoparticles for siRNA delivery:
Evaluation of gene silencing efficiency, intracellular
distribution and exocytosis 127
Chapter 6 Layer-by-layer nanoparticles for glaucoma therapy 157
Chapter 7 Summary and Conclusion 191
Appendix Abbreviations Curriculum Vitae Acknowledgements
Statement in Lieu of an Oath
199
201
203
205
Chapter 1
Hyaluronan as a promising excipient for ocular drug delivery
Published in European Journal of Pharmaceutics and Biopharmaceutics 2017, 113, 34-49
This chapter was published as: M. Guter and M. Breunig, Europ. J. Pharm. Biopharm. 2017,
113, 34-49, doi: 10.1016/j.ejpb.2016.11.035
Abstract
Hyaluronan (HA) is a naturally occurring polysaccharide and well known for its exceptional properties such as high biocompatibility and biodegradability, along with a low immunogenicity. Besides its use for various biomedical applications it recently came into focus as a favorable excipient for the formulation of various ocular therapeutics. This review article summarizes the ocular distribution of HA and its most heavily investigated binding protein “cluster of differentiation 44” (CD44) which is the rationale for the clinical use of HA, primarily as an additive in ocular applications ranging from eye drops to contact lenses.
Moreover, examples will be given for using HA in various pre-clinical approaches to generate
entirely new therapeutics, most notably in the field of nanotechnology.
1 Introduction
Ocular diseases are numerous and diverse. Some present few symptoms or a mild progression, while others may be associated with pain, double vision, inflammation, and ultimately vision loss. In the past decade, significant progress has been achieved toward a better understanding of the pathogenesis and genetics of many ocular diseases such as glaucoma or ocular neovascularization (e.g., age-related macular degeneration (AMD) or diabetic retinopathy (DR) [1]). Over the same time period, the field has seen a substantial expansion of both therapeutic options and routes of delivery. For many years, ocular medicines have been applied predominantly as eye drops. Despite their potency, these medications have a long list of drawbacks, including poor compliance, inadequate application by patients, poor bioavailability, and systemic side effects [2]. In addition, these therapeutic options fail at treating diseases that originate in the posterior segment of the eye. The emergence of biologics has helped fill this therapeutic gap; intra-vitreal injections are now routine procedures [3]. In addition, research and development on novel drug delivery materials and strategies for therapeutics, ranging from small molecules to biologics and nucleic acids, have advanced the field tremendously (for reviews please refer to [4–8]).
More recently, the polysaccharide HA has come into focus as a favorable excipient for the formulation of various ocular drug delivery systems. HA is a naturally occurring structural component of the extracellular matrix (ECM) and can be gained by extraction from animal tissues such as the rooster comb but also via bacterial fermentation in Streptococci or Bacilli [9,10]. Its non-immunogenic, non-fouling and favorable physicochemical properties (e. g.
high water binding capacity, pseudoplasticity, optical transparency) have made the use of HA
in biomedical applications quite popular. For example, it has been applied as an artificial
lubricant in joints [11], a substitute material for the vitreous body [12], and a scaffold material
for tissue engineering [13]. Additionally, HA has contributed to the development of cancer
diagnostics and therapy [14,15]; for instance, HA-based hydrogels have been used for local
delivery of macromolecular drugs (e.g., for interferon- 2 [16,17], trastuzumab [18], and
nucleic acids [19]). HA-drug conjugates have also shown promise when formulated to
improve drug solubility and stability, in a similar fashion to PEGylation [20]. Lastly, HA has
been used successfully as a targeting sequence to guide drug-loaded nanoparticles (NPs) to
their final destination [15]. Despite these efforts, HA-based excipients are just beginning to
garner recognition as powerful catalysts for new drug delivery applications in the field of
ocular pathology.
Here, we first present a general overview of HA and its properties. Then, we describe the distribution of HA and its binding protein CD44 in the anterior and posterior segment of the eye. Finally, we provide a critical review of recent developments in the field of HA- assisted ophthalmic drug delivery and highlight HA’s potential therapeutic effects.
2 HA and its interaction with the CD44 receptor
Figure 1. Chemical structure of HA. HA is a natural heteropolysaccharide composed of the repeating disaccharide unit D-glucuronic acid / N-acetyl-D-glucosamine. The monomers are linked via -1,4- and -1,3-glycosidic bonds until the molecular weight reaches values of up to 107 kDa.
HA is the main component of the ECM and can therefore be found ubiquitously in the human body. The largest amount is present in the skin, which accounts for over 50 % of total body HA [15]. However, the concentration is also high in the vitreous body, from which it was originally isolated in 1934 [21]. Its significant role in the body is underlined by its high synthesis rate and an extremely rapid turnover of 5 g per day in an adult of 70 kg [22].
HA is composed of alternating units of D-glucuronic acid and N-acetyl-glucosamine (Figure 1). The monomers are linked via -1,4- and -1,3-glycosidic bonds and form a long, linear polymer chain. The pK
avalue of the carboxylic acid groups is between 3 and 4 [23].
Consequently, the polysaccharide is negatively charged at physiological pH. Native HA is
present as high molecular weight (MW) material up to 10
7kDa and is well known for being
biocompatible, biodegradable, non-toxic, and non-immunogenic [24]. Although the chemical
structure is very simple, HA exhibits exceptional properties and various biological functions
[25]. Due to its hydrophilic character and long chain length, HA can bind enormous amounts
of water and may thus swell up to a volume 1000-fold greater than its original solid volume
[15,26]. HA solutions are viscous and characterized by shear thinning and pseudo-plastic
flow behavior [27]. Consequently, the polymer is ideally suited as a lubricant in several
biological functions and was rapidly deployed as an artificial viscosupplement, which diminishes friction and abrasion in the joint gap [28].
HA promotes the structure formation and hydration of the ECM throughout various tissues in the body and enables their mechanical functionality and stability [26,29]. Interactions with several extracellular binding proteins (e.g., versican, aggrecan, and neurecan), distributed unevenly across tissues, strengthen the structure of the matrix [30]. In addition, hydration and electrostatic repulsion of anionic carboxyl groups on the polysaccharide chain contribute to the expansion of the polymer [25]. The resulting pressure caused by the swelling is strong enough to separate neighboring tissues, allowing cells to migrate within the newly emptied spaces. This HA-induced “cell migration highway” is of special importance in tissue development, wound healing, and cancer metastasis. Cellular receptors for HA such as CD44, receptor of hyaluronan mediated mobility (RHAMM) and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) link cells to the ECM, mediate cellular mobility, transduce signals from the extracellular environment to the intracellular space and are responsible for HA internalization and degradation. Some HA-binding proteins share a structural domain known as “link module” but other binding sites have also been identified [31].
The most heavily investigated of the HA-binding proteins is the CD44 receptor, which exists in multiple isoforms due to a significant degree of alternative splicing [32]. Glycosylation also broadens CD44 structural diversity and influences receptor activity. Given the polyanionic character of HA, it is at first glance surprising that hydrogen bonding and van der Waals forces dominate the CD44-HA interaction (at least for the murine CD44 receptor), as found by Banerji et al. via co-crystallization [33]. However, the anionic charges are unevenly distributed over the polymer, resulting in a substantial hydrophobic surface along the polyelectrolyte chain. A minimum chain length of six monosaccharides is necessary for monovalent binding of HA to the CD44-receptor [34]. Multivalent binding enhances receptor affinity and can be observed when the polysaccharide chain length exceeds 20 monomers. Mizrahi et al. implemented a model to estimate both, the binding strength between HA and its receptor CD44 and the receptor coverage by HA of different MW [35].
They immobilized a recombinant human CD44-Fc chimera on a surface plasmon resonance
(SPR) sensor chip and evaluated the number of receptors occupied by free HA. Additionally,
they assessed binding of HA-functionalized NPs. Strong binding of free HA was measurable
at or above an HA MW of 132 kDa and coverage was found to match theoretical predictions
(Table 1). In contrast, when the HA-chains were affixed to the NP surface, binding strength
was strongly reduced, most likely due to restricted polymer mobility. However, when the MW was increased to 700 kDa, free and immobilized HA exhibited similar binding characteristics. As these results were achieved using a planar and cell-free model, one should exercise caution when extrapolating results to an in vivo environment.
Table 1. Estimated CD44-Fc coverage by free HA at different MWs. Assuming a receptor density of 3 receptors per 1000 nm² and taking into account the radius of gyration of differently sized HA-chains, the receptor coverage was calculated. With permission taken from [35].
HA MW [kDa]
Radius of gyration [nm]
Area [nm²]
CD44 coverage (CD44 molecules available per HA)
6.4 4 49 1 (0.16)
31 10 327 1
132 24 1709 5-6
700 66 13678 44
1500 105 34365 110
After binding to the CD44 receptor, HA can be internalized via receptor-mediated endocytosis and degraded [25,36]. Subsequently, downstream signaling cascades may be activated, triggering cell survival, migration or inflammation. HA-CD44-interactions thus play a role in physiological and pathophysiological processes like embryonic development or tumorigenesis. Depending on the tissue, the degree of polymerization and the binding proteins involved, HA mediates different – and often opposing – effects as a messenger molecule. Whereas high MW HA has non-immunogenic and anti-proliferative properties, smaller degradation products seem to promote angiogenesis, inflammation and tumor growth [15]. However, very short HA fragments may in turn transduce an entirely different set of signals that effect suppression of tumor growth [26]. The complex interplay between HA, its receptors, synthases and cleaving enzymes, along with the therapeutic concepts based on these findings, are the subject of several review articles [26,37–39]. These investigations do not primarily refer to ocular cells because a deeper analysis and understanding of the CD44 receptor characteristics of primary ocular cells or ocular-derived cell lines, respectively, has to the best of our knowledge not been addressed so far.
It is important to mention that the use of HA is also associated with some drawbacks.
Because HA is of natural origin, variations are possibly more important than they are for
synthetic polymers [40]. Significant batch-to-batch variations may complicate further
downstream processing. But the clinical use of several biopolymers – therapeutically or as an
excipient (e. g. heparin, alginate, protamine or gelatin to name just a few) has demonstrated
that detailed specifications of individual characteristics such as MW, grade of purification or
biological offspring, can diminish differences between batches thereby facilitating further processing. In addition, the production of HA by extraction from animal sources is a common procedure – but contamination with proteins or viruses are possible and might become a problem in certain applications. Equally, the fermentation in bacteria carries the chance of mutation of the bacterial strain and the co-production of toxins or pyrogens [41].
Especially if the usage is not limited to topical administration, the allergic potential and the risk of developing an anaphylactic reaction cannot be denied. Luckily, the microbiological production and purification methods have made tremendous progress in the past few years.
Another point is the pharmacological effect of HA itself. As discussed earlier, depending on the MW and concentration, HA is involved in tumor metastasis, cell migration and proliferation on the cellular level. These effects also depend on the organ and tissue type and are not fully understood yet. Consequently, it is hard to fully estimate the overall effect of HA-based formulations.
3 Anatomy of the eye
Figure 2. Anatomy of the eye. A) General structure of the eye bulb. B) Anterior chamber of the eye. Arrow shows the outflow pathway of the aqueous humor produced in the ciliary body.
C) Cross-section of the back of the eye.
The structure of the eye is unique and provides many advantages for drug delivery, e.g.
immune privilege – the absence of immunological reactions against diverse antigens – and
accessibility for local administration [42]. In the following section, the prevalence of HA and
CD44 in the eye is described to provide support for the notion of using HA as a component in ocular drug delivery formulations.
The eye can be divided into two functional regions as illustrated in Figure 2. The anterior segment makes up approximately one third of the total volume of the eye and comprises the cornea, iris, ciliary body and lens (Figure 2B) [43]. Aqueous humor (AH), which provides nutrients to the cornea and lens, fills the spaces within the anterior segment. It is produced in the ciliary body, flows through the gap between lens and iris into the anterior chamber where it drains through the trabecular meshwork (TM) and Schlemm’s canal into the episcleral vein (arrow in Figure 2B) [44]. By adjusting the AH production and drainage, the intraocular pressure (IOP) is regulated and adapted to guarantee the correct shape – and thus, optimal optical properties – of the eye bulb under changing conditions [45]. Belonging to the posterior segment, the vitreous body represents the largest part of the eye bulb. This clear, highly hydrated gel fills the space between the lens and the retina and serves structural, optical and developmental functions [46]. The back of the eye contains the retina, the choroid and the optic nerve (Figure 2C). The retina is composed of several structures: neuronal ganglia and sensory cells, separated from the vitreous by an inner limiting membrane [47].
Rods and cones, responsible for perception of light/dark and colors, respectively, are two types of photoreceptors found in the retina. Müller glia cells in the neuronal layer stabilize the retina and support the functions of the photoreceptors, for example by providing nutrients, removing metabolic products and regulating neuronal excitability [48]. The retinal pigment epithelium (RPE) is the outermost layer of the retina, and plays an important role in maintaining the viability of the photoreceptors. Among other tasks, the pigmented cells absorb scattered light, protect retinal cells against photo-oxidative stress, transport substances between photoreceptors and choroidal capillaries, and help to regenerate sensory cells [49]. Bruch’s membrane divides the retina from the choroid, which is the vascular layer of the posterior segment of the eye [47]. Blood vessels provide the outer retinal areas with nutrients and oxygen.
Albeit to a varying extent, HA is present in all described structures of the eye and is often found in close proximity to CD44 receptors. To improve the comprehensibility of the following, the distribution of HA and CD44 is described from front to back of the eye.
3.1 Anterior segment of the eye
HA is an integral component of the cornea, typically collocated with its receptor CD44. In
particular, immunostaining revealed high CD44 receptor density in the corneal epithelium
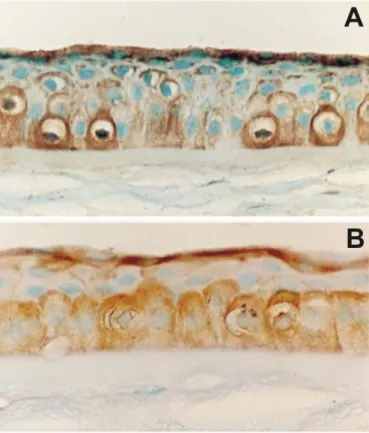
and endothelium, whereas lower density was observed in the hydrophilic corneal stroma (Figure 3) [50,51]. Interestingly, inflammation and other destructive processes affecting the cornea lead to a change of CD44 isotype (alternative splicing) and an increase in receptor density [52–54]. These observations are consistent with the influence of HA on epithelial cell mobility during wound healing; re-epithelialization occurs at a faster rate in the presence of HA [55,56]. In this case, interactions between epithelial CD44 receptors and HA on the ocular surface likely facilitate cell movement along this polysaccharide chains. Additionally, high MW HA seems to have a protective effect in UVB (radiation) exposed corneal epithelial cells due to its inhibitory effect on apoptotic signals and inflammatory cytokines [57]. The HA “reservoir” in tears is produced by corneal epithelial cells [58,59] and contributes significantly to the integrity of the ocular surface.
Figure 3. Central portion of the human cornea illustrating the distribution of HA and CD44 in the corneal epithelium and stroma. A) HA (brown) is localized on the basal and superficial epithelium but hardly in the stroma. Magnification: x 132. B) Intense staining for CD44 (brown) can be detected on the basal and superficial epithelium and is similar to the distribution pattern of HA. Magnification: x 150. Counterstaining with light green. With permission taken from [51].
HA has also been detected and quantified in the AH of enucleated eyes from humans,
mammals and birds [60]. It might be involved in maintaining IOP, as it is capable of binding
large amounts of water and therefore generating a high swelling pressure. IOP regulation is
controlled primarily by varying outflow resistance to AH in the TM. In the TM, HA makes
up 20-25% of the total glycosaminoglycans of the ECM [61] and is mainly associated with the sieve-like networked epithelia of the trabecular beams [62,63] (Figure 2B). HA therefore co-determines the structure of the aqueous outflow pathway and as such is directly involved in regulating IOP. Interestingly, the aqueous HA concentration increases with age in healthy individuals, but is reduced in patients suffering from primary open angle glaucoma (POAG), a severe eye disease characterized by elevated IOP damaging the optic nerve [64,65]. Low HA concentrations are accompanied by an increased level of free CD44s, a soluble degradation product of the CD44 receptor [66], since the quantity of available HA is insufficient to inactivate CD44s. As CD44s – especially the hypophosphorylated form – is toxic to retinal ganglion cells and supporting cells in the prelaminar portion of the optic nerve, it might contribute to the degradation of neuronal cells and vision loss in POAG, provided its concentration exceeds a certain limit [67]. Consequently, Nolan et al. have proposed the use of CD44s as a protein marker for visual field loss in POAG [68].
Finally, HA has been detected in the iris and lens. Similar to observations made in the cornea,
HA concentration and CD44 receptor density have been found to rise upon injury to these
structures [69,70]. Apparently, HA also promotes tissue regeneration in the internal space of
the anterior segment [71,72]. It has been shown via in vitro cell culture that migration of
human lens epithelial cells on laminin or collagen coated plates is dependent on CD44
receptor interactions [73]. However, as very few HA-based therapeutics have been
investigated for this site of application, scant detail is available.
3.2 Posterior segment of the eye
Figure 4. Schematic drawing of the vitreous structure. Collagen fibrils form the hydrogel scaffold. HA immobilizes water within the spaces and leads to stretching of the collagen fibers.
With permission taken from [46].
The vitreous of the eye was the first structure in which HA was detected in 1934 [21]. Today, the distribution and function of HA is well-known; HA is the principal glycosaminoglycan of the vitreous and found in concentrations varying from 65 to 400 µg/mL in humans, increasing with age [74]. A network of collagen fibers of different types forms the scaffold of the vitreous. High MW HA chains fill the spaces between the protein fibers and immobilize large amounts of water. The resulting swelling pressure leads to stretching of the collagen fibers, which mediates the internal tension of the vitreous (Figure 4) [46].
Experimental ex vivo treatment of vitreous with hyaluronidase reduces its water content and
stiffness, but also increases contraction of its collagen fibers. These first two properties
generated clinical interest (including a phase III clinical trial [75]) around use of hyaluronidase
as a liquefaction agent to be applied prior to vitro-retinal surgery, which is often required to
treat retinal detachment or DR. However, intensified traction on retinal structures may increase the risk of exacerbating retinal damage, with the potential for subsequent vision loss [76]. Hyaluronidase also promotes natural HA turnover, and is partially responsible for maintaining the gel structure [77]. Depending on the species, the half-life of vitreal HA is estimated to range from 10 to 70 days. This differs significantly from other tissues, where half-life is no more than one week, and typically less than one day [78]. This extremely long half-life could be due to a combination of high vitreal HA concentration and scarce HA- secreting cells. Thus, faster replacement of the polymer would impair vitreal functionality.
In the back of the eye, Müller glia and cells of the RPE produce extracellular HA for the
retina and the choroid [79,80]. In vitro studies have been conducted to investigate the
synthesis of HA by RPE cells [81]. RPE cells secrete HA preferentially from the apical
surface and are thus responsible for the significant amount of HA present in the
interphotoreceptor matrix. Other cell types are involved in HA homeostasis. Gross-
Jendroska et al. showed that the production of a paracrine factor by fetal RPE stimulates HA
synthesis in neighboring choroidal mesenchymal fibroblasts [82]. The corresponding CD44
receptor can be found on Müller glia microvilli in the retina (Figure 5) [83]. There, the
receptor can be detected during all stages of fetal development and is regarded as a surface
marker for progenitor cells destined to become Müller glia [84,85]. The interactions between
HA and its receptor contribute to structuration of the ECM around photoreceptors and
neurons and enhance the internal strength of the tissue [86,87]. Again, CD44 expression has
been shown to increase after retinal injury. This observation supports evidence of CD44
upregulation in proliferating cells in vitro and informs an understanding of the impact of HA
on healing processes in the cornea and lens, as discussed earlier [88–90].
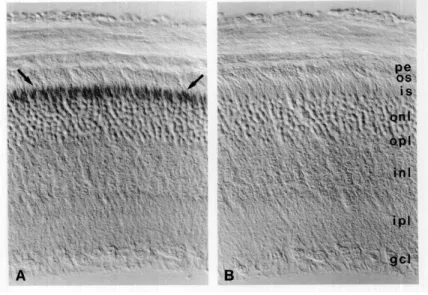
Figure 5. A) Immunoperoxidase labeling on sections of fixed adult mouse retina illustrating the presence of CD44 at the outer limiting membrane and directly adjacent tissue. These structures include the Müller glia microvilli and the lower portion of the photoreceptor inner matrix.
However, discrimination between both structures was not possible. Hence it was not clear if the staining included only one or both components. B) No label was seen if an isotype control was used. pe = pigment epithelium; os = outer segments; is = inner segments; onl = outer nuclear layer; opl = outer plexiform layer; inl = inner nuclear layer; ipl = inner plexiform layer; gcl = ganglion cell layer. Magnification: A x450, B x460, C x500. With permission taken from [83].
HA and CD44 are also involved in pathological alterations of the posterior segment. In particular, the Mochimaru group has investigated the impact of HA and CD44 on the development of choroidal neovascularization (CNV) [91]. CNV is characterized by an abnormally high degree of new blood vessel formation in the choroid. These blood vessels are not restricted to the choroid but rather grow through Bruch’s membrane into the retina.
As vessels developed in this way are prone to bleeding and leakage, the retina can be
damaged, potentially impacting a patient’s vision. Treatment with a hyaluronsynthase-2
inhibitor (4-methylumbelliferone) or with an anti-CD44-antibody reduced CNV-severity by
25% (Figure 6): an impressive result, given that many other factors contribute to the
development of CNV. This observation was explained in reference to the proangiogenic
effect of HA and its potential to recruit macrophages; a high number of HA receptor LYVE-
1-positive macrophages were found in healthy human choroids [92]. Similarly, the interaction
of HA with its receptor CD44 is involved in inflammatory cell migration [93]. In
experimental autoimmune uveoretinitis, a model for serious inflammatory reactions in the
eye, the rolling of CD44 positive leukocytes along the HA-rich endothelium of venules and
postcapillary venules in the retina enables blood cells to cross the blood-retina-barrier. In
this case, too, the administration of anti-CD44-antibodies reduced the severity of
inflammation and showed promise as a potential therapeutic strategy.
Figure 6. Reduction of CNV severity by antibody blockade of CD44. The anti-CD44 antibody was significantly more effective in reducing the CNV volume compared to a non-targeting isotype-control antibody. n = 44 for all. *p < 0.01. With permission taken from [91].
HA fulfills other functions in the choroid. During the process of developing clear and sharp
vision (emmetropization), the choroidal thickness is adjusted to an individual’s requirements
via choroidal deposition of HA and osmotically driven water retention [94–96]. This adjusts
the length of the optical path (distance between lens and retina). Even though the
investigations uncovering this mechanism were performed in chickens, HA is expected to
play a role in this process in humans, as well.
4 Applications of HA in the eye
Figure 7. Possible sites of HA application in the ocular environment. Topical administration of therapeutics by eye drops, lenses or inserts is preferred due to ease of accessibility. However, some substances must be applied by injections into the vitreous cavity or the retina.
Figure 7 summarizes typical therapeutic agent application sites in and on the eye. The topical
route is the preferred mode of administration due to ease of accessibility. This works well
for substances which have their effect in the anterior segment of the eye, such as lubricating
eye drops or glaucoma therapeutics. However, if the drug must reach a structure in the
posterior eye, like the retina, topical administration is ruled out; intravitreal injection is
necessary to bring the therapeutic closer to its target. The broad distribution of HA and its
binding proteins in the eye feature prominently in HA’s potential as a component of new
therapeutic modalities. In this section, we discuss a broad range of applications for HA: as
an excipient to improve the effectiveness of ocular dosage forms, as a therapeutic itself and
as an enabling compound for novel drug delivery approaches. For an overview refer to
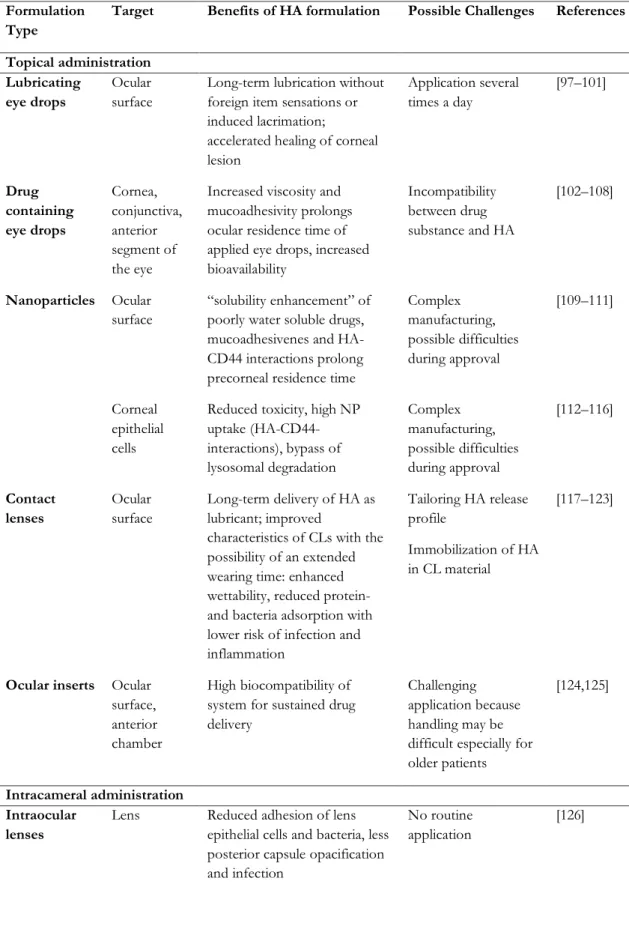
Table 2.
Table 2. Overview of HA-related applications in the eye according to the type of administration.
Formulation Type
Target Benefits of HA formulation Possible Challenges References
Topical administration Lubricating
eye drops
Ocular surface
Long-term lubrication without foreign item sensations or induced lacrimation;
accelerated healing of corneal lesion
Application several times a day
[97–101]
Drug containing eye drops
Cornea, conjunctiva, anterior segment of the eye
Increased viscosity and mucoadhesivity prolongs ocular residence time of applied eye drops, increased bioavailability
Incompatibility between drug substance and HA
[102–108]
Nanoparticles Ocular surface
“solubility enhancement” of poorly water soluble drugs, mucoadhesivenes and HA- CD44 interactions prolong precorneal residence time
Complex manufacturing, possible difficulties during approval
[109–111]
Corneal epithelial cells
Reduced toxicity, high NP uptake (HA-CD44- interactions), bypass of lysosomal degradation
Complex manufacturing, possible difficulties during approval
[112–116]
Contact lenses
Ocular surface
Long-term delivery of HA as lubricant; improved
characteristics of CLs with the possibility of an extended wearing time: enhanced wettability, reduced protein- and bacteria adsorption with lower risk of infection and inflammation
Tailoring HA release profile
Immobilization of HA in CL material
[117–123]
Ocular inserts Ocular surface, anterior chamber
High biocompatibility of system for sustained drug delivery
Challenging application because handling may be difficult especially for older patients
[124,125]
Intracameral administration Intraocular
lenses
Lens Reduced adhesion of lens epithelial cells and bacteria, less posterior capsule opacification and infection
No routine application
[126]
Formulation Type
Target Benefits of HA formulation Possible Challenges References
Intravitreal administration Vitreous
substitutes
Vitreous body
Biocompatibility, easy injection, biodegradability, suitable optical properties
Biodegradation too fast, batch-to-batch variation
[12,127]
Nanoparticles Retina Enhanced mobility in vitreous without impaired interaction with cells;
possibility of specific targeting
Complex
manufacturing, possible difficulties during approval
[128–130]
Retinal administration
Retinal patches Retina Non-toxic, self-adhesive Visit to doctor necessary because application not possible by patients
[131,132]
Hydrogels for protein and stem cell delivery
Retina High biocompatibility, suitable mechanical properties
Still in experimental stadium, no clinical trial so far
[133–135]
4.1 Topical administration
Many medicines are applied topically to the eye, and for good reason. Accessibility, proximity to target structures and a reduced risk of side effects when compared with systemic dosing are all positive features of topical administration. In addition, this application route has economic benefits.
Lubricating and drug containing eye drops
Instillation of eye drops is a procedure which can easily be managed by the patient, reducing costly visits to the ophthalmologist. Unfortunately, not all topical treatments are effective.
Sometimes the active ingredients drain too quickly or are unable to overcome ocular barriers, the latter of which represents a major hurdle for retinal therapeutics in particular [136].
Corneal epithelia and endothelia, corneal stroma, attached mucins or AH flow are just some of the main hurdles that must be traversed with topical application, depending of course on the physicochemical properties of the drug (e.g., partition coefficient and MW).
Nevertheless, eye drops remain a valuable method for ocular drug delivery.
Lubricating eye drops (e. g. HyloVision®, ArtelacSplash®, Hyabak®) are one of the most
prominent applications for HA in the ocular environment. Several artificial tear solutions
utilize HA as a viscosity-enhancing and mucoadhesive excipient for the treatment of dry eye disease (DED). The world prevalence of DED is approximately 6 to 34 %, and an age over 50 years and being female are known risk factors [137]. Patients are affected by ocular irritation, pain and transient visual impairment [138] caused by tear film instability, increased hyperosmolarity of the tear film and inflammation of the ocular surface [139]. The first-line therapeutic option is supplementation with artificial tears to lubricate the eye, replace missing tear fluid and normalize tear film osmolarity [140,141]. Viscous HA solutions or higher concentrated hydrogels are frequently applied to slow drainage of the eye-drop formulation, prolonging its therapeutic effect [142,143]. Compared to other excipients, such as polyvinyl alcohol or celluloses, the main advantage of high-viscosity HA solutions is their shear- thinning behavior [144]. Consequently, foreign item sensations and induced lacrimation, unpleasant symptoms themselves, are avoided.
To quantify the moisturizing potential of eye drops, one can measure tear film thickness.
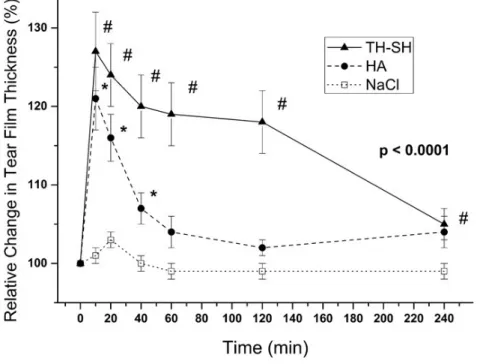
Following application of a 0.15 % HA formulation, tear film thickness was elevated for up to 40 minutes. Supplementation with 3 % trehalose increased tear film thickness by 30 % compared to the baseline value and extends the duration of the effect for up to 3 hours (Figure 8) [100,101]. These features could lead to decreased eye drop application frequency with no loss of therapeutic effect, representing a substantial benefit for the patient.
Figure 8. Relative tear film thickness as a surrogate parameter for the moisturizing potential of different types of eye drops. (TH-SH: 3 % trehalose + 0.15 % HA, HA: 0.15 % HA, NaCl: 0.9 % NaCl). HA-containing eye drops are able to stabilize the tear film for 40 minutes. With addition of trehalose, the effect can be extended for three hours. With permission taken from [101].
HA solutions have also been investigated as a treatment for pathologically elevated tear fluid osmolarity, regarded as a key factor in the pathogenesis of DED. Troiano et al. compared two unpreserved 0.4 % HA eye drops, one of them isotonic (300 mosmol/l), the other hypotonic (150 mosmol/l) [99]. After a treatment period of one week, hypotonic eye drops were more effective at reducing the symptoms of dry eye compared to isotonic drops. Rose Bengal staining also showed that hypotonic drops improved the vitality of corneal epithelial cells. Importantly, two thirds of the treated patients preferred the hypotonic formulation at the end of the study.
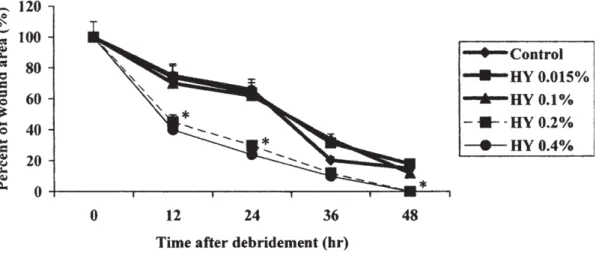
HA eye drops have also demonstrated additional beneficial effects in the treatment of pathological alterations in DED. In particular, an impressive correlation between the velocity of corneal lesion healing and the concentration of the instilled HA solution was reported in rabbits (Figure 9) [97]. Relative to a 0.1 % HA solution, healing was accelerated by a factor of 2 within 24 hours when 0.2 % HA was applied. It seems like improvement of epithelial cell migration and proliferation is a dose-dependent and therefore a pharmacological effect.
Additionally, viscosity and lubrication of the eye drops increase with higher HA concentrations, reducing mechanical damages like friction or abrasion during blinking to the ocular surface. Investigations in diabetic rats have arrived at similar results [98].
Figure 9. In vivo effects of HA (800 – 1400 kDa) on corneal epithelium wound healing. Eye drops were applied directly after corneal denudation of rabbits and again 2, 4, 6, 24, 28 and 30 hours later. Wound closure was measured by staining with 1 % fluorescein. n=4, p=0.05.
Control: 0.9 % NaCl-solution. With permission taken from [97].
Beside its usage as a wetting and lubricating agent, HA is also used as a viscous,
mucoadhesive excipient in general to prolong ocular residence time and reduce the systemic
drainage of drug-containing eye drops [106,107]. For example, HA was used to increase the
bioavailability of anti-glaucoma parasympathomimetic pilocarpine eye drops in rabbits [108].
Bioavailability of the drug, measured as the area under the curve (AUC) for miosis (pupil constriction) after topical instillation, was increased with increasing HA concentrations in the formulation. The prolongation of drug response, rather than an amplification of its effect, confirms similar findings found in previous studies [102]. Additionally, increasing the MW of HA applied led to lower concentrations of the excipient being required to obtain the same pupil-constricting effect, e. g. 0.75 % for 1600 kDa HA but only 0.125 % for 4600 kDa HA [108].
Bernatchez et al. have investigated the potential of HA in regards to enhancing the bioavailability of the antibiotic gentamicin in eye drops [105]. In comparison to buffered aqueous solution, the concentration of gentamicin in the tear fluid was increased by a factor of 2 for at least ten minutes upon inclusion of HA in the formulation. While these characteristics would not reduce application frequency, the increased concentration might improve the corneal penetration and bioavailability of the drug, enhancing its therapeutic efficacy. Herrero-Vanrell et al. have described the mucoadhesive properties and surface tension of corneal mucin and HA along with other excipients such as carboxymethylcellulose or polyacrylic acid; they then combined these polymers with the antimuscarinic drug tropicamide and investigated its bioavailability and mydriatic potential [103]. HA was found to increase the bioavailability by 70% relative to the excipient-free drug solution mainly due to prolongation of the mydriatic response. The effect was explained by reference to the similar properties of mucus and HA, as acidic groups of the polymers interact with the sialic parts of ocular mucins in a comparable fashion to mucus glycoproteins itself. Since tropicamide is used as a short-term mydriaticum, the benefit of using HA in formulations of this drug is limited. However, the results may be generalized and applied to other therapeutic substances with higher clinical relevance.
In general, increasing the solution viscosity is a common approach for prolonging the ocular
residence time of eye drops. The usage of in situ gelling excipients represents a particularly
elegant approach, combining the simple administration of common low-viscosity eye drops
with the favorable features of a more viscous formulation. Poloxamers or polyethylene
glycol-polypropylene glycol block co-polymers are a frequently used class of thermogelators
that may be used for this purpose; gelation occurs upon warming to body temperature as the
hydrophilicity and hydration of the polyethylene part decreases, enabling stronger
hydrophobic interactions and the formation of poloxamer micelles. The volume fraction of
micelles increases and the liquid becomes a gel when it exceeds a certain limit [145]. Cho et
al. combined the thermogelation of poloxamer 407 with the mucoadhesivenes of HA by
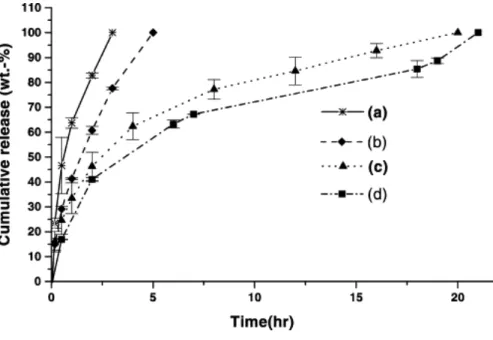
grafting both polymers with EDC/NHS chemistry (a technique for linking two educts via their reactive esters with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N- hydroxysuccinimide) [104]. The two polymers’ properties combine to extend drug release profiles, as shown below for the model substance ciprofloxacin (Figure 10). An HA amount of at least 7 % by weight must be exceeded to observe a relevant effect.
Figure 10. Release profile of ciprofloxacin from in-situ-gelling poloxamer 407-HA-hydrogels.
(a) poloxamer 407 alone (b) graft copolymer (HA, 1.18 wt.%), (c) graft copolymer (HA 6.87 wt.%) and (d) graft copolymer (HA 13.99 wt.%), all with 1.75 wt.% ciprofloxacin. With permission taken from [104].
NP formulations
NP formulations represent another therapeutic opportunity to take advantage of HA’s unique properties. Development of HA-modified NPs for topical ocular instillation has been motivated by two end goals. The first is – again - prolongation of ocular residence time for a drug substance. Drainage of NPs is slower than that of common eye drop formulations because they are able to interact with the corneal epithelium. Second, NPs can serve as a delivery vehicle for entry of various therapeutics into corneal cells. If the NP composition is chosen carefully, cellular specificity and the degree of cellular uptake can be enhanced, for example by leveraging NP-cell surface receptor interactions.
If a drug substance is poorly water soluble, extension of the precorneal residence time is of special importance, as it provides a straightforward and easy way to enhance bioavailability.
An excellent example is cyclosporine A (CyA), an immunosuppressive drug used to increase
tear production in patients with DED. Loading of CyA into NP composed of benzalkonium
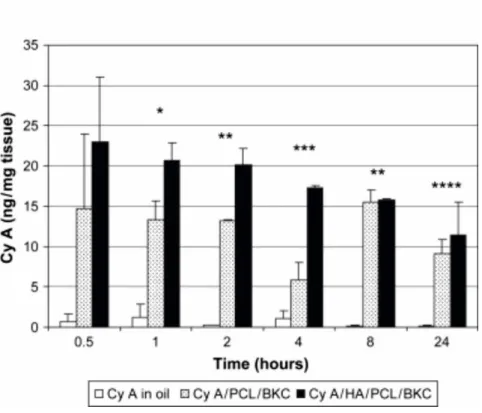
chloride and poly-ε-caprolactone boosted corneal CyA concentration by a factor of 6 to 8 compared to that achieved with a castor oil formulation (Figure 11) [111]. This effect was attributed amongst others to the formulation as a NP and the presence of benzalkonium chloride. Corneal CyA concentration was even higher for HA-modified NP, possibly due to increased mucoadhesion and interactions with corneal CD44 receptors.
Figure 11. CyA concentration in rabbit corneas. 25 µl of CyA formulation was applied four times with a delay of ten minutes in between applications to both eyes of New Zealand rabbits.
Samples were obtained following the last instillation. Corneal CyA levels were significantly higher for the NP formulation composed of poly-ε-caprolactone and benzalkonium chloride (CyA/PCL/BKC) compared to that of the castor oil formulation (CyA in oil). If the NPs were modified with HA 1000 kDa (CyA/HA/PCL/BKC), corneal CyA accumulation exhibited further increase. With permission taken from [111].
Similar results concerning corneal CyA concentrations were observed for nano-sized
microgels formed in situ [110]. Thermoresponsive N-isopropylacrylamide was grafted to
11 kDa HA to combine the unique properties of both polymers. An increase in temperature
to a value greater than the critical solution temperature of the polymer, upon application of
the eye drops, led to the formation of microgels. Corneal CyA levels reached their maximum
value two hours after application and were 6-fold higher than those of the castor oil
formulation. Ibrahim et al. have also demonstrated use of HA’s mucoadhesive properties
[109]. They prepared NPs from Eudragit, a commercial available polymer for the fabrication
of sustained release dosage forms, and loaded them with gatifloxacin and prednisolone.
These NPs were able to release their antibiotic and anti-inflammatory payloads over a period of up to ten hours in vitro , depending on their composition. In order to benefit clinically from this sustained drug release, NPs must also remain on the ocular surface for an extended period of time. Consequently, the authors coated their NPs with HA. With this HA-modified NP formulation, the corneal bioavailability of gatifoxacin was increased to 28 µg*hour/g compared to only 3.6 µg*hour/g for commercial eye drops.
Instead of using NPs to simply retard lacrimal drainage of a drug substance, they can also serve as a vehicle for delivering diverse therapeutic agents into corneal epithelial cells. In order to succeed as an intracellular drug delivery vehicle, NPs must reach their target cells without being hindered by components of the ocular surface. Additionally, they must be stable until entering the cell, at which point they must be able to release their cargo. Hornof and de la Fuente, for example, exploited HA as a targeting moiety for delivery of genetic material into cells of the cornea and conjunctiva [115]. In general, complexation of DNA with polyethyleneimine (PEI) yields acceptable transfection efficiencies due to the cationic charge of the complexes and their endosomolytic properties. However, blood proteins or ECM-components as proteoglycans or glycoproteins tend to adsorb onto the complexes and limit their ability to interact with cell membranes. By coating the complexes with low MW HA, nonspecific binding of proteins and other anionic compounds was reduced and stability of the complexes was increased. Despite the negative surface charge of the HA-coated complexes, and their concomitantly reduced affinity to cell membranes, uptake and transfection efficiency into human corneal epithelia cells was similar to the uncoated control.
In vitro , beta-galactosidase expression was increased by a factor of 10 to 15 compared with naked DNA. This result is surprising, as positively charged particles, such as uncoated polyplexes (complexes of anionic nucleic acids and polycations), are known to be significantly more effective at transfection than neural or anionic particles. By antibody- mediated blockade of CD44 on the cellular surface, the group also showed that uptake must be a receptor-mediated process.
In addition, the therapeutic potential of DNA-loaded HA-chitosan NPs was analyzed in vitro
[116]. Here, higher HA content increased transfection efficiency in proliferative ocular cell
lines (human corneal and conjunctival epithelia). Again, a connection between NP uptake
and CD44 binding was found. In vivo experiments demonstrated the efficacy of this
formulation after topical application to rabbit eyes [114]. Beside receptor-specific
interactions, the general mucoadhesive properties of HA might promote NP uptake after
topical application. At therapeutic doses, in vitro toxicity of this delivery system is exceedingly low [116]. Tolerability and safety was further confirmed in vivo [112].
A key benefit of incorporating HA into this type of NP formulation is its influence on the mechanism of cellular entry and intracellular targeting [113]. Often, particles are trapped within endosomes and lysosomes after internalization. This might cause degradation of the NP cargo and a loss in functionality. However, HA-chitosan-oligomer NPs were internalized by a receptor-mediated, caveolin-dependent internalization process in vitro , bypassing the cellular compartments dedicated to degradation and resulting in high transfection efficiencies for ocular surface epithelial cells.
Macroscopic drug delivery systems
Beside working on eye drops and NPs, efforts have been made to develop drug delivery systems in order to achieve a sustained liberation and dissolution of drug substances to the cornea and conjunctiva.
Hume et al. succeeded in producing HA-based films for sustained delivery of the anti-
inflammatory steroid prednisolone [125]. They esterified HA with varying amounts of benzyl
alcohol to obtain polymers with different degrees of hydrophilicity that were suitable for
producing drug-loaded films. Higher degrees of esterification resulted in enhanced
hydrophobicity and diminished polymer swelling capacity. HA derivatives with the highest
hydrophobicity (100 % of carboxyl groups esterified) enabled the best drug release
characteristics, with constant lacrimal prednisolone levels of 45-72 µg/mL over 24 h in
rabbits. The Calles group developed HA-itaconic acid films, crosslinked with polyethylene
glycol diglycidyl ether, that show excellent material properties and good biocompatibility in
vitro and in vivo [124]. Timolol, a substance which reduces the production of AH in the ciliary
body and lowers IOP, was physically incorporated into the film during manufacturing. A
therapeutic effect on IOP could be observed for more than ten hours, as opposed to eight
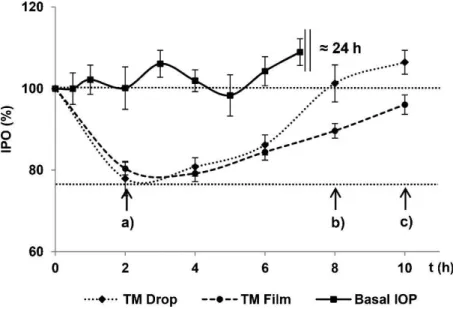
hours for the conventional eye drop formulation (Figure 12). Since this finding is significant
but clinically not relevant, it remains to be seen whether the lengthened time of therapeutic
impact is worth the difficult handling of the insert in this specific case.
Figure 12. IOP of rabbits after the administration of timolol as an eye drop formulation (TM Drop) or as an ocular insert (TM Film). The lowest IOP was observed two hours after application for both formulations (a). After eight hours, IOP reached the baseline value in the eye drop group (b) whereas it took additional two hours in the insert group (c). With permission taken from [124].
Possibly more common – as already known and applied by millions of people for vision correction - are contact lenses (CL). It has been shown that this approach is not only suitable for the sustained delivery of small MW drugs but also for polymers such as HA [121,122].
An example is the strategy of biomimetic, molecular imprinting as shown in Figure 13A
[123]. Here, modified Nelficon A – a polyvinyl alcohol derivative – was crosslinked with UV-
crosslinking monomers. The particular monomers used were chosen because of their
chemical similarity to amino acids found at the HA-binding site of CD44. The hypothesis
was that these monomers would generate a template for directed interactions between the
hydrogel scaffold and the HA-polymer within the CL when the crosslinking reaction takes
place in presence of HA. HA would have higher affinity for such “memory sites”, which
would slow down release. Using this approach, CL with an HA dissolution profile of
6 µg/hour over 24 hours was achieved, matching therapeutic doses. While such CL are
suitable for long-term supply of the ocular surface with HA, incorporation of HA into CL
can be applied more generally to improve wettability of the lens material and increase the
comfort to wear. Other groups pursued this goal and fixed HA within the CL material, either
by creating a crosslinked HA network within the CL, as in Figure 13B [117,120], or by
covalent grafting of HA to the polymeric CL compounds, as in Figure 13C [118]. Beside
higher hydrophilicity and wettability, this resulted in decreased adsorption and denaturation
of proteins on the CL surface. Depending on the strategy of HA functionalization, the
sorption of lysozyme was reduced to only 5 to 70 % compared to that of the unmodified
control. This reduces the risk of developing inflammatory eye diseases [119] and provides for the opportunity of an extended wearing time.
Figure 13. Immobilization of HA in CL material. A) Molecular imprinting; directed interactions between HA and “memory sites” of the hydrogel scaffold. B) HA-crosslinking; fixation of HA due to convolution of individually crosslinked HA- and polymer network. C) Covalent linkage;
immobilization of HA by covalent grafting to the polymer.
4.2 Intracameral administration
Related to the aforementioned CL, Wang et al. extended HA incorporation to produce modified intraocular lenses (IOL), which are used for cataract patients [126]. IOLs are artificial lenses that substitute the natural lens if it becomes cloudy or damaged. Common side effects of the surgical implantation procedure are opacification of the new lens as well as inflammation and infection. To overcome these effects, Wang et al. covalently grafted an HA-lysozyme composite to the surface of the IOL, providing it with anti-adhesive and antibacterial properties. HA modification appeared to reduce adsorption of lens epithelial cells and prevented opacification. Additionally, fewer bacteria adhered to the implant compared with the unmodified lenses. Lysozyme, a lytic enzyme which is naturally present in tear fluid, kills remaining bacteria and therefore prevents the spread of microorganisms, reducing the risk of developing sight-threatening infections. The efficacy of this approach was measured via fluorescence microscopy.
4.3 Intravitreal administration
Vitreous substitution
HA has also been extensively investigated as a vitreous substitute. Vitreous substitution is
required during vitro-retinal surgery or in cases where the retina detaches from the RPE, as
in DR. Conventional tamponade agents are inert gases, silicon oil or perfluorocarbon liquids [146–148]. However, these materials have significant disadvantages. Gases work for just a short period during and after surgery as they are rapidly absorbed by the bloodstream.
Additionally, they might cause sight-threatening elevations of IOP under certain conditions.
Due to their toxicity, perfluorocarbon liquids are used only during the surgical intervention itself, and not afterward. Silicon oils are suitable for long-term vitreous replacement but must eventually be removed, as glaucoma, cataracts and other complications have been reported.
During this procedure, retinal re-detachment can also be induced. HA is a promising substitute as a result of its good biocompatibility – it is the principal component of the vitreous humor – its easy injectability and its biodegradability. Unfortunately, the process of degradation happens very rapidly, as total vitreal HA is replaced every 10 to 70 days (see
“anatomy section”) [78]. In response, HA derivatives with slower biodegradation kinetics were developed.
Crosslinking of HA with adipic dihydrazide or N-vinyl-pyrrolidinone / UV light reduced biodegradation in vitro to 10 % within the first four weeks, and with even slower degradation kinetics after that point [12]. UV-crosslinked hydrogels did not show any signs of toxicity in cell culture experiments and were well-tolerated after implantation in the vitreous cavity of rabbits. The vitreous substitutes were stable for more than six weeks and their optical and mechanical properties remained almost unchanged. Grafting of a thermogelling poloxamer F127 to HA resulted in a vitreous substitute that was readily injectable at room temperature and with properties reasonably well-matched to those of the vitreous upon gelation at 37 °C [127]. However, these graft copolymers were unable to provide the needed rheological and mechanical characteristics nor to slow degradation kinetics required for long term use [146].
As already outlined, a general problem of all biomaterials of natural origin – including HA- based vitreous substitutes - is a significant batch-to-batch variation. Therefore, synthetic polymers such as polyethylene glycol (PEG) derivatives, polymethacrylates and polyurethanes have been developed for this purpose. They combine reasonable rheological and refractive properties with high reproducibility and consistent quality.
NP formulations
If therapeutics must reach the posterior segment of the eye, application via eye drops is
unsatisfactory, as the drug is required to cross several barriers and traverse relatively long
distances [149]. Intravitreal injection represents one possibility for specific administration to
this difficult to access area of the eye. Even then, applied substances must diffuse through
the vitreous cavity to reach their target. Xu et al. investigated the general factors influencing NP mobility in the vitreous body by multiple particle tracking on a fluorescence microscope [150] and found dependencies on NP colloidal stability, particle size, surface charge and concentration. Neutral, PEGylated NPs that tend not to interact with components of the vitreous humor are able to diffuse freely up to a size of 510 nm (Figure 14). Negatively charged NPs are hampered in their mobility if they are larger than 200 nm or if the concentration exceeds a certain threshold. Cationic particles in turn are fully immobile, independent of size or concentration. They stick to anionic vitreal collagen fibrils via electrostatic interactions and might only be considered therapeutically if drug release at the site of injection is required.
Figure 14. Influence of surface charge, size and concentration of NPs on their mobility in the vitreous body. Electrostatic, steric and hydrophobic effects influence the diffusivity of NPs in the vitreous gel. PEGylated particles (PS-PEG) are able to diffuse freely up to a size of 500 nm.
Anionic NPs (PS-COOH) smaller than 200 nm move unimpeded. If their size or concentration increases, diffusivity is reduced due to steric and hydrophobic effects. Cationic polystyrene- amine NPs (PS-NH2) interact electrostatically and aggregate within the vitreous. With permission taken from [150].
PEGylation of NPs reduces aggregation and nonspecific binding to ECM components and
enhances the diffusivity of particles in the vitreous [151]. Unfortunately, in addition to
reducing undesirable interactions, interactions with target cells and tissues are also reduced
[152]. A possible solution could be the modification or coating of nanostructures with HA
to enhance NP mobility in the vitreous without reducing cellular uptake or transfection
efficiency. The movement of different types of NPs through the vitreous and retina was
investigated by Koo et al. [130]. HA-NPs of about 200 nm in size with a negative surface
charge of -26 mV diffused through the vitreal gel and accumulated in deeper regions of the
retina following intravitreal injection. PEI-modified NPs (+ 33 mV) with a slightly larger size
were almost completely immobile. Interestingly, the HA-NPs seemed to be eliminated from
the posterior segment within a few days, which might be a beneficial property for candidate
therapeutics. Further detailed investigations concerning the mobility and functionality of
polyplexes were carried out by Martens et al. [129]. Martens determined the diffusion
coefficients of polyplexes functionalized with HA of varying molecular weight by fluorescent
particle tracking microscopy in excised bovine eyes. The unmodified probe (108 nm,
+29 mV) reflects the reduced mobility of positively charged complexes in the vitreous
(Figure 15). Diffusivity increased upon functionalization with HA and displayed the most
improvement when modified with 137 kDa HA. Although the particles became larger
(343 nm), the negative surface charge of -30 mV and the antifouling properties of HA seem
to adequately compensate for the increase in size. HA 2700 kDa was not suitable as a
diffusivity enhancer here, as the resulting particles became too large and aggregated.
Figure 15. Diffusion coefficients of polyplexes obtained by single particle tracking analysis in intact bovine vitreous. The strongest increase in diffusivity was found for polyplexes coated with HA 137 kDa (HA137-pplxs); no improvement was achieved with HA 2700 kDa (HA2700- pplxs). The black, solid line represents the bimodal distribution of diffusion coefficients of uncoated, cationic polyplexes. The mobilities of the respective NP in HEPES-buffer are shown as dotted lines. With permission taken from [129].
Further in vitro experiments confirmed both the polyplexes’ ability to enter RPE cells via interactions with CD44 and their superlative cell transfection capabilities. Similar results were achieved by Gan et al. [128], who modified chitosan-lipo-NP (chitosan-NP capped with a lipid bilayer) with HA of different MW. The modified NP were able to diffuse freely through the vitreous and accumulated on the inner limiting membrane following intravitreal injection.
In a model of retinal damage – experimental autoimmune uveitis – the group found that
these HA-modified chitosan-lipo-NP were even able to specifically target the RPE, as CD44
is upregulated in this disease.
4.4 Retinal administration
Retinal patches
During aging, compounds of the vitreous body become liquefied and collagen fibrils change their structure, forming bundles and becoming stiffer [153]. The tension on the retina increases and begins to peel the sensitive structure from the back of the eye, which may lead to impaired vision or blindness. Patients suffering from diabetes typically show faster liquefaction and run a higher risk of retinal detachment [154]. Holes and breaks in the retina accelerate the shedding, allowing vitreous fluid to leak into the subretinal space [155].
Therefore, it is critically important to seal retinal breaks and prevent the accumulation of fluids. Common options for the treatment of such breaks are laser-induced retinal fixation or the administration of anti-inflammatory steroids. Another possibility is the application of gel-like retinal patches. Sueda et al. developed an absorbable material based on HA and carboxymethylcellulose to close retinal breaks [132]. The patch was liquid-tight and stayed in place for 30 days on retinas of excised bovine eyes. No signs of toxicity were observed in vivo . Unfortunately, its application during vitrectomy surgery is quite difficult. In order to enhance the therapeutic effectiveness of retinal patches, 1,2,3,4-diepoxybutane crosslinked HA gels were prepared and loaded with triamcinolone acetonide [131]. This combined the retina stabilizing effect of a self-adhesive patch with the local delivery of an anti- inflammatory agent. The gel was directly applied to the retinal injury via an injector system, a special kind of syringe. While the application process was still challenging, the patch was well-tolerated during initial trials and the approach is a promising therapeutic option to reduce retinal inflammation and accelerate healing.
Hydrogels for protein and stem cell delivery