Z. Kristallogr. NCS 215 (2000) 4 8 3 ^ 8 4 483
© by Oldenbourg Wissenschaftsverlag, München
Crystal structure of 4,6-dinitroresorcinol, C6H 4 N 2 06
T. Kolev
1, M. Berkei
1, C. Hirsch
1, H. Preut*
1, P. Bleckmann
1and V. Radomirska
11 I Universität Dortmund, Fachbereich Chemie, Otto-Hahn-Str. 6, D-44221 Dortmund, GermanyII Bulgarian Academy of Science, Institute of Organic Chemistry, 1113 Sofia, Bulgaria Received November 9, 1999, CCDC-No. 1267/330
Abstract
C6H4N2O6, monoclinic, P 1 2 , / c l (No. 14), a = 11.709(2) A,
b = 5.030(1) A, c = 12.587(3) A , P = 91.27(3)°, V = 741.1 A3, Z = 4 , Rgt(F) = 0 . 0 3 4 , wRKf(F2) = 0 . 0 9 2 , T= 2 9 3 K .Source of material
The title compound was obtained by boiling 2.5 g of 1,3-di- chloro-4,6-dinitrobenzene (Lancaster, Eastgate England) in a 500 ml In KOH under reflux for 6 hours. A f t e r the cooling of the dark-red solution it was acidified with conc. HC1 to give a orange-yellow precipitate. The resulting 4,6-dinitroresorcinol was isolated and recrystallized twice from ethanol and after that from ethylacetate; mp 480 K - 482 K (mp 478 K - 479 K [ 1 ]). The pu- rity of the compound was confirmed by elemental analysis, IR, UV-vis and mass spectrometry.
Discussion
An increasing interest in organic materials with high optical nonlinearity has emerged in receant years due to potential appli- cations in photonics, optoelectronics, optical data storage and op- tical information processing. The design of nonlinear optical (chromo)phores exhibiting enhanced molecular quadratic (or cu-
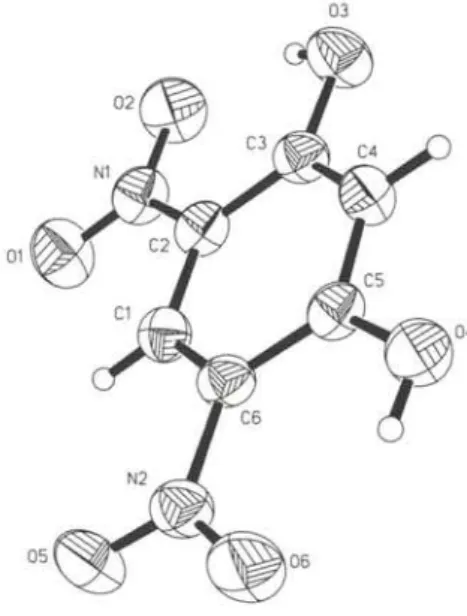
bic) responses is a requisite for a design of a efficient second- and third order nonlinear optical materials [2, 3,4]. The structure de- termination was undertaken in order to obtain more structure in- formation about 4,6-dinitroresorcinol - a new class of two-dimen- sional charge transfer system. The molecular geometry and intra- molecular hydrogen bonding of 4,6-dinitroresorcinol was studied by means of gase-phase electron diffraction [5], In the crystal, which is held together by a system of hydrogen bonds, the mole- cule is nearly planar whereas in the gas phase it has a torsion angle C - C - N - 0 of 14.4°. In both states there exists two strong intramolecular O—H---0 hydrogen bonds.
Table 1. Data collection and handling.
Crystal: pale yellow, parallelepiped, size 0.3 x 0.3 x 0.35 mm Wavelength: Mo Ka radiation (0.71069 À)
1.65 cm"1
Diffractometer, scan mode: Nonius Kappa CCD, 635 frames (Ato = 1 °) 2x 20 s per frame
26max:
49.98°N(hkl)mcasured, N(hkl)unique:
4604,1293
Criterion for I0bS, Nfhkl)^:/obs > 2 a(/obs), 1048
N(param)refme<t'- 144Programs: PARST [6], SHELXS-97 [7],
SHELXL-97 [8], SHELXTL-plus [9]
Table 2. Atomic coordinates and displacement parameters (in Â2).
Atom Site X >• z t/iso
H(l) Ae 0.839(1) -0.272(3) -0.050(1) 0.047(4) H(2) Ae 0.634(1) 0.426(3) 0.0805(9) 0.039(3) H(3) 4e 0.526(1) 0.151(3) -0.140(1) 0.074(6) H(4) Ae 0.868(1) 0.304(3) 0.212(1) 0.053(5)
* Correspondence author
(e-mail: uch002@uxplh.htz.uni-dortmund.de)
484
4 , 6 - D i n i t r o r e s o r c i n o lTable 3. Atomic coordinates and displacement parameters (in Â2).
Atom Site x y z U11 U22 U33 U12 U13 t/23 C ( l ) 4e 0.7972(1) -0.1372(3) -0.0239(1) 0.0370(7) 0.0343(7) 0.0336(7) -0.0005(6) 0.0019(6) 0.0010(6) C(2) 4e 0.6982(1) -0.0585(3) -0.07515(9) 0.0384(8) 0.0376(8) 0.0287(7) -0.0049(6) -0.0031(6) 0.0014(6) C(3) 4e 0.6341(1) 0.1573(3) -0.0359(1) 0.0324(7) 0.0396(8) 0.0340(8) 0.0002(5) -0.0052(5) 0.0059(5) C(4) 4e 0.6737(1) 0.2860(3) 0.0541(1) 0.0397(8) 0.0363(8) 0.0374(8) 0.0049(6) -0.0014(6) -0.0013(6) C(5) 4e 0.7747(1) 0.2148(3) 0.10575(9) 0.0360(7) 0.0351(7) 0.0302(7) -0.0046(5) -0.0024(5) 0.0015(5) C(6) 4e 0.8360(1) -0.0051(2) 0.06557(9) 0.0306(7) 0.0381(7) 0.0324(7) -0.0015(6) -0.0040(5) 0.0053(6) 0 ( 1 ) 4e 0.71842(9) -0.3905(2) -0.19886(8) 0.0703(7) 0.0555(7) 0.0484(6) 0.0072(5) -0.0061(5) -0.0167(5) 0 ( 2 ) 4e 0.57354(9) -0.1307(2) -0.21671(8) 0.0588(7) 0.0654(8) 0.0477(6) 0.0008(5) -0.0240(5) -0.0063(5) 0 ( 3 ) 4e 0.53686(8) 0.2460(2) -0.08105(8) 0.0435(6) 0.0574(7) 0.0467(6) 0.0108(5) -0.0156(5) -0.0043(5) 0 ( 4 ) 4e 0.80777(9) 0.3613(2) 0.18949(7) 0.0441(6) 0.0452(6) 0.0416(6) 0.0018(5) -0.0118(5) -0.0078(4) 0 ( 5 ) 4e 0.99204(8) -0.2823(2) 0.07704(8) 0.0451(6) 0.0550(7) 0.0556(7) 0.0160(5) -0.0042(5) -0.0031(5) 0 ( 6 ) 4e 0.98119(8) 0.0333(2) 0.19136(8) 0.0541(7) 0.0595(7) 0.0545(7) 0.0068(5) -0.0263(5) -0.0083(5) N ( l ) 4e 0.66211(9) -0.2035(2) -0.16938(9) 0.0468(7) 0.0448(7) 0.0347(6) -0.0044(6) -0.0056(5) -0.0018(5) N(2) 4e 0.94241(9) -0.0926(2) 0.11406(8) 0.0365(6) 0.0428(7) 0.0376(7) 0.0005(5) -0.0052(5) 0.0045(5)
Acknowledgments. We thank the National Science Foundation (Bulgaria) grant X-605 and the 'Internationales Büro bei der DLR' (Germany) for the support of our project BUL-006-99. One of us(Ts.K.) thanks the Alexander von Humboldt Stiftung for financial support.
References
1. Beyerlein, F.: Untersuchungen über die Frage, ob das Benzoaxazol zu den naphtoiden oder den benzoiden Bizyklen gehört. Dissertation, TH Braunschweig, Germany, 1933.
2. Nalwa, H. S.; Miyata, S. (Eds.): Nonlinear Optics of Organic Molecules and Polymers. CRC Press, Boca Raton 1997.
3. Wolff, J. J.; Wortmann, R.: Organic Materials for Second-Order Non-Linear Optics. In: Advances in Physical Organic Chemistry, Vol. 32, (Editor D. Bethell), p. 121-217, 1999.
4. BosshardC.;Sutter, K.; Pretre,P.; Hulliger,J.;Flôrsheimer, M.; Kaatz,P.;
Giinter, P.: Organic Nonlinear Optical Materials. Gordon & Breach, Am- sterdam 1995.
5. Borisenko, K.; Zauer, K.; Hargittai, I.: Intramolecular Hydrogen Bonding and Molecular Geometry of 4,6-Dinitroresorcinol from Gas-Phase Elec- tron Diffraction. J. Phys. Chem. 99 (1995) 13808-13813.
6. Nardelli, M. A system of Fortran routines for calculating molecular struc- ture parameters from the results of crystal structure analyses. J. Appl.
Crystallogr. 28 (1995) 659.
7. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
8. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures, University of Gôttingen, Germany 1997.
9. Sheldrick, G. M.: SHELXTL-Plus. Release 4.1 Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA 1991.