MHc/class‑ii‑positive cells inhibit corticosterone of adrenal gland cells in experimental arthritis: a role for IL‑1β, IL‑18, and the inflammasome
Volltext
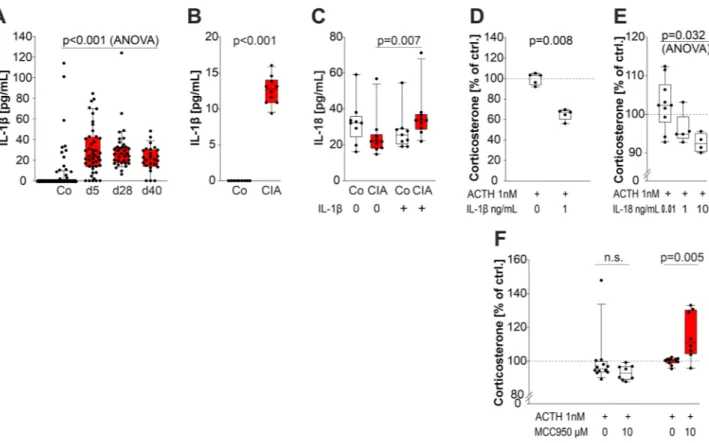
Abbildung


ÄHNLICHE DOKUMENTE
Zusammenfassend konnte gezeigt werden, dass IL-22 in diesem Modell kontextabhängig sowohl protektive Eigenschaften in der Leberregeneration aufweist als auch eine
Die Gene, die IL-1 α , IL-1 β und IL-1Ra kodieren, liegen beim Mensch in einem Genlocus auf dem Chromosom 2 und für einige Kombinationen dieser Gene wurde bereits
First experi- ments were performed to test our hypothesis that CCL che- mokines that attract monocytes inhibit the ATP-dependent release of IL-1 β by LPS-primed human monocytic
Analysis of IL-6 release, COX-2 expression, and IκB degradation reveals that IFNγ can not be regarded as a general inhibitor of IL-1β action on A549 cells.(A) A549 cells were
Kürzlich konnte gezeigt werden, dass sowohl Hausstaubmilben- allergene als DAMPs [51] als auch Hämolysine und bakterielle Lipoproteine von Staphylokokkus aureus als PAMPs fungieren
But the data obtained with the dominant negative IL-10 receptor α- chain mouse model revealed that IL-10 is not essential for the differentiation of mature and functional T R 1
Litterarum obligatio древня го нрава. Литтеральное обязательство Юстиніанова права. Emtio venditio, locatio conductio, societas, mandatum. Pacta adjecta,
Collectively, our data reveal the importance of the two IL-1 family members IL-1 and IL-36 for the control of systemic fungal infection by induction of protective