Fact Sheet No: 30/3
MINI tracka II
In-situ Miniature Fluorimeter
MINItracka II augments
Chelsea’s renowned range of
AQUAtracka submersible
fluorimeters. The emphasis on the different variants of MINItracka II is on low cost, size and power consumption - whilst still retaining excellent sensitivity, without sacrificing the all important turbidity rejection. All Chelsea's fluorimeters are notable for their ability to make measurements in high levels of daylight.
INTRODUCTION General
The MINItracka Fluorimeter is the latest in-situ optical sensor from Chelsea Technologies Group.
Using a single high intensity LED light source, surface mount electronics and an acetyl pressure housing, the MINItracka is extremely compact, with very low power consumption. The stability of the LED light source provides a long re-calibration interval. Particular design attention has been paid to the rejection of ambient daylight. The highly successful cowl design of the UV AQUAtracka has been incorporated as an integral feature of the MINItracka pressure housing, removing the need to pump sea water through a dark chamber. Hence, when using MINItracka, no water flow corrections are needed for subsequent calculations.
SPECIFICATION Optical
Chlorophyll-a Chlorophyll-a Rhodamine Amido
Rhodamine Fluorescein Excitation wavelengths 430/30 nm 470/30 nm 470/30 nm 425/30 nm 480/80 nm Emission wavelength 685/30 nm 685/30 nm 590/45 nm 550/30 nm 530/30 nm Concentration range 0.03-100 µg/l 0.03-100 µg/l 0.03-100 µg/l 0.04-200 µg/l 0.03-100 µg/l Resolution 0.01 µg/l 0.01 µg/l 0.01 µg/l 0.025 µg/l 0.01 µg/l Calibration standard Chlorophyll-a in
acetone Chlorophyll-a in acetone
Nephelometer Phycoerythrin Phycocyanin Excitation wavelengths *470/30 nm 530/30 nm 590/35nm
Emission wavelength *470/30 nm 580/30 nm 645/35 nm
Concentration range *0.04-100 FTU 0.03-100 µg/l 0.03-100 µg/l
Resolution *0.01 FTU 0.01 µg/l 0.01 µg/l
Mechanical Electrical
Body Size 149mm long x 70 mm dia Input Voltage 7 to 40 VDC
Weight in Air 0.7 Kg Output Voltage 0 to 4 VDC(linear)
Weight in Water 0.15 Kg Power requirements 0.7 W typical
Pressure Housing Acetal ‘C’ Signal : Noise 10,000 : 1 @ full scale Depth Rating 600 metres
Connector Subconn MCBH4M
149 mm
70 mm 4
3 2 1 Locating
Pin
1 Power -ve 2 Signal +ve 3 Power +ve 4 Signal -ve
OPERATION
MINItracka is an extremely simple instrument to use. There are only four connections to be made, two each for power and signal lines. The pins on the four way connector on the end cap of MINItracka (as shown in the preceding section) should be connected as follows. The colours of the corresponding cores of the cable supplied with the unit are also listed, as this is more relevant for most users.
1 Power Negative Black Cable 2 Signal Positive White Cable 3 Power Positive Red Cable 4 Signal Negative Green Cable
When a voltage between 7 and 40 Volts DC is supplied to MINItracka, a signal voltage of between
0 and 4 Volts is returned, corresponding to the determinant concentration. Each unit is individually set in this range of concentrations. Calibration constants are listed on the individual calibration sheet enclosed with each instrument. When measuring chlorophyll, or other primary production breakdown products, the user is recommended to calibrate MINItracka for in vivo use, as fluorescence response varies considerably between phytoplankton species.
Although MINItracka will work with loads as low as 2 kohm, it is recommended that the data logger used has as high an input resistance as practical to keep cable resistance effects to a minimum. MINItracka will drive cables of capacitance up to 3uF but this does not obviate the need for proper data telemetry where ‘good practice’ normally dictates it.
CALIBRATION Chlorophyll
In Vivo Chlorophyll. The MINItracka excitation light source uses a wavelength selected, narrow band Blue LED so as to minimise the variation in excitation peak wavelength from instrument to instrument. This in turn minimises the variation in instrument to instrument response to in vivo chlorophyll-a compared to that of chlorophyll-a in acetone which is used in production to characterise the MINItracka.
Of course, the fluorescence response of in vivo chlorophyll varies with the species of phytoplankton, the ambient conditions etc., so the scaling factor between chlorophyll-a in acetone and in vivo chlorophyll also varies. For this reason the user is recommended to carry out a calibration using the species of phytoplankton most likely to be encountered in deployment.
Factory Characterisation & Data Output Interpretation. During production, MINItracka is set- up to give nominally 3.5 ± 0.5 Volt output when exposed to 100µg/l of chlorophyll-a in acetone, and typically has an rms noise of 0.5mV when electrically filtered as suggested in section 6. MINItracka is linear over the range 0.03 - 100µg/l chlorophyll-a in acetone and is provided with a “laboratory calibration certificate” detailing the Scaling Factor and Offset under these conditions. Thus, having determined the offset and scaling factor, Equation 1 below can be used to determine the chlorophyll-a concentration:
Chlorophyll-a Concentration [µg/l] = (Vsample - Voffset) x Scale Factor - Equ.1 where;
Vsample = MINItracka voltage output (VDC) when measuring the sample
Voffset = MINItracka voltage output (VDC) when measuring the water with no chlorophyll present.
User in vivo Calibration. It is recommended that the user carries out an in vivo calibration to determine the Scaling Factor and offset voltage (no chlorophyll blank) in order that the MINItracka voltage output can meaningfully be converted into in vivo chlorophyll concentrations.
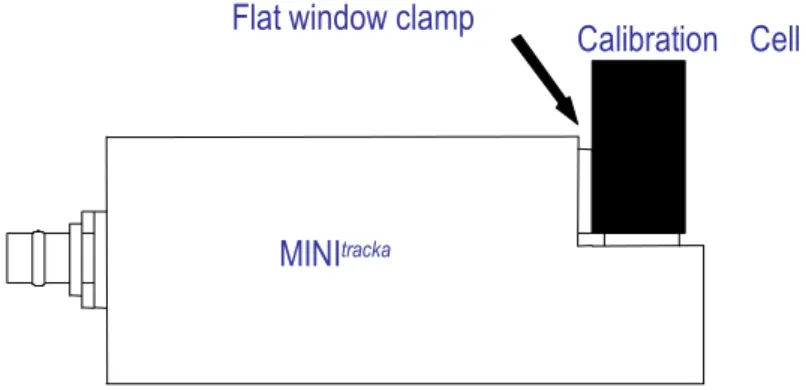
This user calibration can be carried out using the calibration cell as shown below, or, more simply, by deploying the MINItracka in a bucket of sea water with a known in vivo chlorophyll concentration. If the bucket approach is used, be sure to suspend the MINItracka unit well away from the sides, just in case the bucket itself has a fluorescence response. (Note: for chlorophyll in acetone calibrations, the calibration cell should always be used, as direct contact with acetone will damage MINItracka)
MINItracka
Calibration Cell Flat window clamp
Figure 1 - Calibration Set-up
Measurements should be taken for a known concentration of in vivo chlorophyll (Vsample), and for water containing no chlorophyll (Voffset). The Scale Factor, simply µg/l per Volt, is determined by manipulation of Equation 1 above, such that;
Scale Factor [µg/l per Volt] = Chlorophyll Concentration [µg/l] - Equ.2 (Vsample - Voffset)
The Scale Factor determined for in vivo chlorophyll measurements is generally much smaller than that seen for chlorophyll-a in acetone.
Performance Check
During production, MINItracka is set-up to give nominally 3.5 ± 0.5 Volt output when exposed to 100µg/l of chlorophyll-a in acetone. The user can check the MINItracka performance against the factory calibration/characterization certificate by making a single point measurement of a sample of known chlorophyll-a concentration (in acetone) and the determination of the acetone voltage offset. NB: this procedure is intended to check that the MINItracka is performing as factory set and cannot be used directly for in vivo chlorophyll determination.
The following equipment is required, and the procedure undertaken in clean working conditions.
• Bottle of pure water.
• 100µg/l Chlorophyll-a (in acetone) standard solution.
• Acetone.
• Calibration Cell (available from Chelsea Technologies Group).
• 100µl calibrated micropipette.
• Stirrer.
• 100 ml volumetric flask.
NOTE - the following precautions must be observed
• It is vital that the calibration cell and stirrer are kept scrupulously clean and dry. They should be cleaned using acetone and dried with lint free tissue.
• New micro pipette tips should be used for each calibration in order to avoid cross contamination.
• The Fluorimeter is affected by ambient light, particularly fluorescent lighting, so it is recommended that it is placed in a dark receptacle when calibrating.
• Never allow solvents to come into contact with the housing or windows of MINItracka which will be immediately damaged.
Procedure
• Remove the light baffle and replace the sloping detector window clamp with the flat window clamp supplied.
• Ensure that the instrument is clean and dry and that the Fluorimeter windows are cleaned thoroughly. NOTE: DO NOT use a PERSPEX® SOLVENT and only use lint free tissue.
• Clean the calibration cell, then add 10 ml of analar grade acetone to the cell. Place the cell on and against the Fluorimeter windows as illustrated earlier. Note the output reading (Voffset
)
• Replace the pure acetone with 10 ml of the 100µg/l Standard Chlorophyll-a solution and note the new reading (Vsample).
Applying the values of Vsample and Voffset to the calibration/characterization equation supplied with the MINItracka should yield a chlorophyll-a concentration in agreement with that of the standard solution placed in front of the MINItracka. The Scaling Factor and Voffset may change with use, though this is expected to be minimal over the life time of the instrument and insignificant through the period of a single deployment/cruise.
Chlorophyll-a Stock Solution (1000µµµµg/l) & Standard Solution (100µµµµg/l)
A chlorophyll-a Stock Solution of 1000µg/l can be prepared by dissolving 1 mg of Chlorophyll- a in 1000 ml of acetone. Nominal 1 mg phials of Chlorophyll-a extracted from spinach may be obtained from Sigma Chemical Co. Ltd., Fancy Road, Poole, Dorset, BH17 7NH, United Kingdom.(Product Code 5753).
NOTE: Phials supplied by Sigma only contain a nominal 1 mg, with the actual weight varying by up to 20%.
An approximate 1000µg/l solution can be prepared as follows:
• First remove any labels from the phial and clean the exterior of the phial with acetone and a lint free tissue. Snap the neck of the phial and drop both pieces into a clean 1000 ml volumetric flask.
• Add 100 to 200 ml of Analar grade acetone to the flask and gently swirl to dissolve the Chlorophyll-a. Make up to the 1000 ml graduation with more acetone and continue swirling for a few minutes, with the stopper in place.
• Store the completed stock solution below 0oC and in the dark. Small amounts (10 to 20 cm-3) should be decanted and brought up to room temperature for use.
A more accurate stock solution can be prepared as follows, if the user has access to a micro balance:
• First remove any labels from the phial and clean the exterior of the phial with acetone and a lint free tissue. Snap the neck from the phial and weigh to 10-microgram resolution.
• Empty contents into a clean 1000ml volumetric flask.
• Re-weigh the phial to within 10 micrograms and note the difference from the original measurement.
• Add 100 to 200 ml of Analar grade acetone to the flask and gently swirl to dissolve the Chlorophyll-a.
• Adjust the subsequent volume of acetone added to the volumetric flask so as to make the solution up to 1000µg/l strength.
A 100µg/l Chlorophyll-a Standard solution can be made from the 1000µg/l stock solution by making up 10ml of stock solution to 100ml with pure acetone.
Rhodamine
The factory calibration is determined using Rhodamine WT diluted in pure water and this will not necessarily ensue for Rhodamine WT in sea water.
Checks to ensure the instrument is performing as factory set can be carried out using a calibration cell as shown below or, more simply, by deploying the MINItracka in a bucket of water with a known Rhodamine_WT concentration. If the bucket approach is used, be sure to suspend the MINItracka unit well away from the sides, just in case the bucket itself has a fluorescence response.
Nephelometer
The factory calibration is determined using Formazin prepared to BS 6068 part 2.13, suspended in pure water. Since scatter is inherently highly dependent on optical wavelength and on particle size, shape and refractive index, it is very difficult to intercompare results from other nephelometers and/or other turbidity standards.
Checks to ensure the instrument is performing as factory set can be carried out using a calibration cell as shown below or, more simply, by deploying the MINItracka in a container of water with a known turbidity/FTU. If the container approach is used, be sure to suspend the MINItracka unit well away from the sides to avoid any scatter from the walls.
Amido Rhodamine
The factory calibration is determined using Amido Rhodamine diluted in pure water; this figure will not necessarily be obtained in sea water.
The user can check the MINItracka performance against the factory calibration/characterization certificate by making a single point measurement of a sample of known Amido Rhodamine concentration (in pure water) and the determination of the water voltage offset. NB: this procedure is intended to check that the MINItracka is performing as factory set.
Phycocyanin
In Vivo Phycoerthyrin. The MINItracka excitation light source uses a wavelength selected, narrow band green LED so as to minimise the variation in excitation peak wavelength from instrument to instrument. This in turn minimises the variation in instrument to instrument response to in vivo Phycoerthyrin compared to that of Phycoerthyrin in PH-7 buffer solution which is used in production to characterise the MINItracka.
Of course, the fluorescence response of in vivo Phycoerthyrin varies with the species of phytoplankton, the ambient conditions etc., so the scaling factor between Phycoerthyrin in PH-7 buffer solution and in vivo Phycoerthyrin also varies. For this reason the user is recommended to carry out a calibration using the species of phytoplankton most likely to be encountered in deployment.
Factory Characterization & Data Output Interpretation. During production, MINItracka is set- up to give nominally 3.5 ± 0.5 Volt output when exposed to 100µg/l of Phycoerthyrin in PH-7 buffer solution, and typically has an rms noise of 0.5mV when electrically filtered as suggested in section 6. Minitracka is linear over the range 0.03 - 100µg/l Phycoerthyrin in PH-7 buffer solution and is provided with a “laboratory calibration certificate” detailing the Scaling Factor and Offset under these conditions. Thus, having determined the offset and scaling factor, Equation 1 below can be used to determine the Phycoerthyrin concentration:
Phycoerthyrin Concentration [µg/l] = (Vsample - Voffset) x Scale Factor - Equ.1 where;
Vsample = MINItracka voltage output (VDC) when measuring the sample
Voffset = Minitracka voltage output (VDC) when measuring the water with no Phycoerthyrin present.
User in vivo Calibration. It is recommended that the user carry out an in vivo calibration to determine the Scaling Factor and offset voltage (no Phycoerthyrin blank) in order that the
Minitracka voltage output can meaningfully be converted into in vivo Phycoerthyrin
concentrations. This user calibration can be carried out using the calibration cell as shown below, or, more simply, by deploying the Minitracka in a bucket of sea water with a known in vivo Phycoerthyrin concentration. If the bucket approach is used, be sure to suspend the Minitracka unit well away from the sides, just in case the bucket itself has a fluorescence response. (Note:
for Phycoerthyrin in PH-7 buffer solution calibrations, the calibration cell should always be used)
MINItracka
Calibration Cell Flat window clamp
Figure 1 - Calibration Set-up
Measurements should be taken for a known concentration of in vivo Phycoerthyrin (Vsample), and for water containing no Phycoerthyrin (Voffset). The Scale Factor, simply µg/l per Volt, is determined by manipulation of Equation 1 above, such that;
Scale Factor [µg/l per Volt] = Phycoerthyrin Concentration [µg/l] - Equ.2 (Vsample - Voffset)
The Scale Factor determined for in vivo Phycoerthyrin measurements is generally smaller than that seen for Phycoerthyrin in PH-7 buffer solution.
Performance Check. During production, Minitracka is set-up to give nominally 3.5 ± 0.5 Volt output when exposed to 100µg/l of Phycoerthyrin in PH-7 buffer solution. The user can check the Minitracka performance against the factory calibration/characterization certificate by making a single point measurement of a sample of known Phycoerthyrin concentration (in PH-7 buffer solution) and the determination of the PH-7 buffer solution voltage offset. NB: this procedure is intended to check that the Minitracka is performing as factory set and cannot be used directly for in vivo Phycoerthyrin determination.
The following equipment is required, and the procedure undertaken in clean working conditions.
• Bottle of pure water.
• 100µg/l Phycoerthyrin (in PH-7 buffer solution) standard solution.
• PH-7 buffer solution.
NOTE - the following precautions must be observed
• It is vital that the calibration cell and stirrer are kept scrupulously clean and dry..
• New micro pipette tips should be used for each calibration in order to avoid cross contamination.
• The Fluorimeter is affected by ambient light, particularly fluorescent lighting, so it is recommended that it is placed in a dark receptacle when calibrating.
• Never allow solvents to come into contact with the housing or windows of Minitracka, which will be immediately damaged.
Procedure
• Remove the light baffle and replace the sloping detector window clamp with the flat window clamp supplied.
• Ensure that the instrument is clean and dry and that the Fluorimeter windows are cleaned thoroughly. NOTE: DO NOT use a PERSPEX® SOLVENT and only use lint free tissue.
• Clean the calibration cell, then add 10 ml of analar grade PH-7 buffer solution to the cell.
Place the cell on and against the Fluorimeter windows as illustrated earlier. Note the output reading (Voffset )
• Replace the pure PH-7 buffer solution with 10 ml of the 100µg/l Standard Phycoerthyrin solution (see below) and note the new reading (Vsample).
Applying the values of Vsample and Voffset to the calibration/characterization equation supplied with the Minitracka should yield a Phycoerthyrin concentration in agreement with that of the standard solution placed in front of the Minitracka. The Scaling Factor and Voffset may change with use, though this is expected to be minimal over the life time of the instrument and insignificant through the period of a single deployment/cruise.
Phycoerthyrin Stock Solution (1000µµµµg/l) & Standard Solution (100µµµµg/l) 1 mg phial of Phycoerthyrin (supplied by Sigma Chemicals).
500 ml of Pure Water.
Buffer Capsules 7.0 Ph x 5.
A clean 100 ml Volumetric Flask.
Glass Stirrer.
Glass Funnel 200ml Glass Cell Filter Paper 1ml Glass Pipette
Measure out 500ml of pure water and add the contents of 5 buffer capsules (PH-7). Allow the capsules to dissolve thoroughly until the water is free from all Particles. Using the glass funnel with filter paper, pour a small amount of the prepared solution into the glass cell and gently rinse. Repeat this 2 more times and shake dry. Repeat this rinsing procedure for the 100ml volumetric flask. Using the glass funnel and filter paper, measure out 100ml of the prepared solution and pour into the glass cell. Remove all labels from the phial and carefully wipe clean.
Pour the entire contents of the phial into the cell and rinse thoroughly until all Phycoerthyrin is dispensed. Decant the resultant liquid into a clean flask. The flask now contains a solution of Phycoerthyrin 10-5
(10,000ug/l ). Sign and date.
Preparation of Calibration Sample is then undertaken by dispensing 1ml of the Phycoerthyrin solution (10-5 ) into the volumetric flask and top up to the line with prepared buffer solution. The flask now contains a Phycoerthyrin solution of 10-7 (100ug/l). Sign and date.
Phycoerthyrin
The calibration procedure is the same as Phycocyanin except that Phycoerthyrin is used instead of Phycocyanin.
Fluorescein
The factory calibration is determined using fluorescein diluted in pure water, (this figure will not necessarily be obtained in sea water.
Checks to ensure the instrument is performing as factory set can be carried out using a calibration cell as shown below or, more simply, by deploying the Minitracka in a container of water with a known Fluorescein in pure water.. If the container approach is used, be sure to suspend the Minitracka unit well away from the sides to avoid any scatter from the walls.
CIRCUIT DESCRIPTION
LED Sample
Precision Current Generator
Clock Photodiode
Amplifiers
Phase Sensitive Detector
DC Amp (Time Const
= 1 sec) Line
Driver Sig Hi
Sig Lo
0 V + V
0 V +5 V
-5 V
Internal Supplies DC-DC
Convertor
The sample is illuminated by a high intensity LED driven by a clock modulated precision current source. If the specimen contains the determinant, then it radiates a shifted signal with the same modulation as the LED. The modulated optical emission from the specimen is detected by a large area photodiode. The signal from the photodiode undergoes several stages of amplification before passing through a phase sensitive detector (p.s.d.), which uses the LED clock signal as its reference. The p.s.d. output feeds into an amplifier with a built in time constant. This determines the overall response time (and noise bandwidth) of the system.
Finally the signal reaches the cable driver and thence the outside world.
The cable driver can power capacitive loads up to 3µF without going into oscillation. While this in could, in theory, permit the use of some
10 kilometres of typical oceanographic cable, proper telemetry should be used over long distances to preserve signal integrity.
The output driver/buffer provides at least 2 mA of signal current, thus ensuring that the +4V full- scale output is available for any load of
≥2 kilohms. The output resistance of the unit is <1Ω, which limits the reduction in calibration due to load to 0.05% at 2 kΩ load.
The whole assembly is powered through an isolating DC-DC converter. The isolation this provides breaks the troublesome, and frequently data corrupting ‘ground loops’ that can be caused by common connections of a number of instruments to a single power supply.
Connecting Minitracka into a suite of sensors is therefore very simple.
LEDs and semiconductors are generally temperature dependent devices. However, careful selection and balancing of components leads to readings from Minitracka having a typical calibration temperature dependence of 0.05% per degree when operating between -5 and +35 centigrade.