ζ . Kristallogr. NCS 213 (1998) 757-758 7 5 7
© by R. Oldenbourg Verlag, München
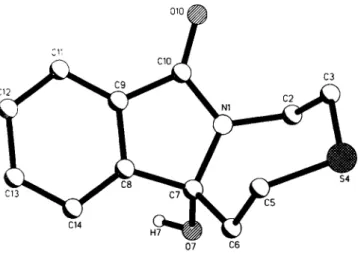
Crystal structure of 4b-hydroxy-4b,6,8>9-tetrahydro-5H-7-thia-9a-aza- benzo[a]azulen-10-one, C12H13NO2S
K. Peters, E.-M. Peters
Max-Planck-lnstitut für Festkörperforschung, Heisenbergstraße 1. D-70506 Stuttgart. Germany
M. Oelgemöller and A. G. Griesbeck
Universität zu Köln. Institut für Organische Chemie, Greinstraße 4, D-50939 Köln, Germany Received April 7, 1998, CSD-No. 409289
Table 1. Parameters used for the X-ray data collection
Source of material: The title compound was prepared from potassium 3-(2-phthalimidoethylsulfanyl)propionate by irradiation (300 nm, 24 h) in an acetone / water mixture (7:3). Extraction and recrystallization from acetone yielded 25% of colorless crystals (see ref. 1). This reaction was studied in context with regioselectivity problems (see ref. 2) and state-selectivity effects (see ref. 3) in ^-phthaloyl cysteine photochemistry. Photodecarboxylation is the dominant process for product formation in the case of potassium carboxylates (see ref. 4) even in the presence of thioethers as competing electron donors. The intermolecular hydrogen bridge 0 7 - H 7 · · 0 1 0 leads to a zigzag chain parallel [100].
C 1 2 H 1 3 N O 2 S , monoclinic. Ce (No. 9), a =6.612(1) A, b =17.600(2) Â, с =9.759(1) Â, β =96.76(1)°, V=1127.8 Â ^ Ζ =4, R(F) =0.041, ÄwCF; =0.041.
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Crystal: colorless lath, size 0.4 χ 0.9 χ 0.1 mm Wavelength: Mo Ka radiation (0.71073 A)
μ: 2.70 cm"'
Diffi^tometer: Siemens P4
Scan mode: ω
^measurement' 293 К
2θηωχ: 55°
S(hkl)unique: 1406 Criterion for Fo". Fo>3a(F„) Ы(рагсап),фа1Г. 147
Program: SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in A^)
Atom Site X У ζ l/iso
H(2A) 4a 0.0997(6) 0.1820(2) -0.1342(4) 0.08 H(2B) 4a 0.0092(6) 0.1085(2) -0.0741(4) 0.08 H(3A) 4a 0.0864(6) 0.1854(2) 0.1087(4) 0.08 H(3B) 4a 0.2550(6) 0.1224(2) 0.1225(4) 0.08 H(5A) 4a 0.6185(6) 0.1474(2) 0.0213(4) 0.08 H(5B) 4a 0.7130(6) 0.2283(2) 0.0081(4) 0.08 H(6A) 4a 0.4434(6) 0.2377(2) -0.1914(4) 0.08 H(6B) 4a 0.6559(6) 0.2020(2) -0.2095(4) 0.08 H(7) 4a 0.31(1) 0.104(3) -0.406(6) 0.09(2) H(ll) 4a 0.5627(7) -0.1157(2) -0.0939(4) 0.08 H(12) 4a 0.8554(8) -0.1270(2) -0.2074(5) 0.08 H(13) 4a 0.9647(7) -0.0260(2) -0.3375(5) 0.08 H(14) 4a 0.7848(6) 0.0904(2) -0.3533(4) 0.08
Atom Site X У ζ Uu {/22 1/33 Un ί/13 t/23
S(4) 4a 0.39823 0.23848(6) 0.08405 0.0528(5) 0.0460(5) 0.0529(5) -0.0078(5) 0.0167(4) -0.0213(5) N(l) 4a 0.2837(5) 0.0927(2) -0.1329(3) 0.034(1) 0.028(1) 0.036(1) 0.001(1) 0.005(1) -0.001(1) C(2) 4a 0.1301(6) 0.1385(2) -0.0761(4) 0.031(2) 0.044(2) 0.047(2) 0.003(1) 0.006(2) -0.006(2) C(3) 4a 0.2022(6) 0.1656(2) 0.0698(4) 0.041(2) 0.053(2) 0.048(2) -0.003(2) 0.015(2) -0.014(2) C(5) 4a 0.5916(6) 0.1984(2) -0.0107(4) 0.039(2) 0.036(2) 0.047(2) -0.005(2) 0.009(2) -0.008(2) C(6) 4a 0.5335(6) 0.1959(2) -0.1666(4) 0.050(2) 0.026(2) 0.041(2) -0.002(2) 0.014(2) 0.001(1) C(7) 4a 0.4273(6) 0.1228(2) -0.2247(3) 0.039(2) 0.029(2) 0.033(2) 0.005(1) 0.008(1) 0.001(1) 0(7) 4a 0.3287(5) 0.1429(2) -0.3557(3) 0.062(2) 0.036(1) 0.032(1) 0.014(1) -0.001(1) 0.002(1) C(8) 4a 0.5699(6) 0.0552(2) -0.2306(4) 0.040(2) 0.027(1) 0.028(1) 0.003(1) 0.001(1) -0.001(1) C(9) 4a 0.5060(6) -«.0048(2) -0.1545(4) 0.042(2) 0.032(2) 0.030(1) 0.001(1) -0.000(1) 0.000(1) C(10) 4a 0.3253(6) 0.0199(2) -0.0899(4) 0.035(2) 0.032(2) 0.034(2) -0.005(1) -0.000(1) 0.002(1)
758
C12H13NO2S ТаЫе 3. (Continued)Atom Site X У ζ t/ll U22 f/33 Í/12 Un t/23
CK 10) 4a 0.2284(5) -0.0149(2) -0.0095(3) 0.050(2) 0.044(1) 0.049(1) -0.004(1) 0.011(1) 0.010(1) C ( l l ) 4a 0.6089(7) -0.0740(2) -0.1454(4) 0.059(2) 0.029(2) 0.042(2) 0.003(2) 0.001(2) 0.001(1) C(12) 4a 0.7799(8) -0.0803(2) -0.2133(5) 0.062(3) 0.038(2) 0.051(2) 0.017(2) 0.000(2) -0.008(2) C(I3) 4a 0.8459(7) -0.0199(2) -0.2907(5) 0.047(2) 0.052(2) 0.048(2) 0.010(2) 0.007(2) -0.012(2) C(14) 4a 0.7408(6) 0.0488(2) -0.3004(4) 0.044(2) 0.039(2) 0.035(2) 0.000(2) 0.007(2) -0.005(1)
References
1. Oelgemöller, M.: Dissertation planned for 1998. University of Cologne, Geimany.
2. Giiesbeck, A. G. ; Hirt, J.; Kramer, W.; Dallakian, P.: Photochemistry of
^-phthaloyl cysteine, its methyl ester, and C-unprotected S-alkyl deriva- tes. Tetrahedron 54 ( 1998) 3169-3180.
4.
5.
Griesbeck. A. G.; Hirt, J.; Peters, K.; Peters, E.-M.; von Schnering, H. G.;
Photo electron transfer induced cyclization of A'-phthaloyI cysteine derí- vales: multiplicity-controlled regioselective CH-activation. Chem. Eur. J.
2 ( 1 9 % ) 1388-1394.
Griesbeck, A. G.; Henz, Α.; Kramer, W.; Nerowski, F.; Oelgemöller, M.:
Peters, К.; Peters, E.-M.: Synthesis of medium- and large-ring compounds initiated by photochemical decarboxylation of (o-phthalimido alkylcar- boxylates. Helvetica Chim. Acta 80 (1997) 912-933.
Sheldrick, G. M.: Program Package SHELXTL-plus. Release 4.1.
Siemens Analytical X-Ray Instruments Inc., Madison (Wl 53719), US A 1990.