Year 10 Chemistry, Mr Blaurock – Worksheet 6
1.5Saturated Hydrocarbons: Isomers
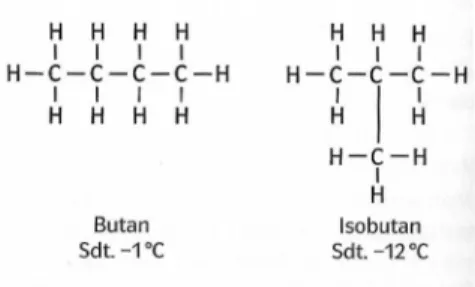
On the last worksheet we saw an interesting thing: there are two alkane molecules with the exact same molecular formula: C4H10. To differentiate the two we call the straight chain molecule n-butane, the other i-butane (iso = equal).
Figure 1: Lewis structures of n-butane and i-butane
As the excerpt from a German book illustrates the two molecules actually differ in their
__________________________. Other properties, e.g. their flammability or chemical reactivity, are quite similar.
Two molecules that share the same molecular formula but are not identical we refer to as _____________________. In this case the atoms in the two molecules are joined together in different ways. These molecules we refer to as ______________________________________ (or ________________________________, dt. Konstitutionsisomere).
Task:
1) Name molecule 1 pictured below. State whether molecule 2 is a constitutional isomer or not and give reasons.
Molecule 1 Molecule 2
2) Draw all possible isomers of n-pentane.
Year 10 Chemistry, Mr Blaurock – Worksheet 6
Drawing organic molecules
Since organic molecules can become quite large in size, it can often be quite tedious and confusing to draw a Lewis structure with each and every chemical bond and hydrogen atom. Also, when drawing three-dimensional Lewis structures the structure can become quite convolutes, especially when lone electron pairs are involved. To address these problems there are several different ways to draw the same organic molecule.
The Lewis structure
The basic type of structural formula, we draw all atoms, bonds and lone electron pairs with a 90°
angle between the bonds/lone electron pairs.
The condensed formula
To reduce the effort in drawing a Lewis-structure all hydrogen atoms that share a bond with a carbon atom are grouped together, only the bonds between the carbon atom groups are drawn.
turns into
(in an organic formula R is a placeholder for any continuation of the carbon chain) By convention we write the hydrogen atoms in a group to the right of the carbon atom.
Draw the complete Lewis structure and condensed formula of heptane:
Three-dimensional Lewis structure and Natta projection (skeletal formula)
When drawing the three-dimensional Lewis structure for an alkane molecule the convention is to draw the longest alkane chain on the plane of the paper with only the hydrogen atoms either sticking out or retreating behind.
The Natta projection or skeletal formula differs from the three-dimensional Lewis structure in two ways. For one the hydrogen atoms in a molecule no longer appear in the formula at all. Secondly the element symbol is no longer used for carbon atoms. We basically only draw the lines representing the bonds between carbon atoms (C-C-bonds) and carbon atoms are found at the intersections of these lines.
Draw the three-dimensional Lewis structure and Natta projection of i-butane (build a model if you need help!):
Figure 2: Natta projection of hexane, the first and third carbon atoms of the chain are marked
Year 10 Chemistry, Mr Blaurock – Worksheet 6