T cell antigens for diagnostic and vaccination purposes
DISSERTATION ZUR ERLANGUNG DES
DOKTORGRADES DER NATURWISSENSCHAFTEN (DR. RER. NAT.) DER FAKULTÄT FÜR BIOLOGIE UND VORKLINISCHE MEDIZIN
DER UNIVERSITÄT REGENSBURG
vorgelegt von
Richard Kiener
aus
Neustadt a.d. Waldnaab
im Jahr
2017
Das Promotionsgesuch wurde eingereicht am:
26.10.2017
Die Arbeit wurde angeleitet von:
Prof. Dr. Ralf Wagner Unterschrift:
Richard Kiener
The results section of this thesis comprises 3 chapters, the second and third of which have been submitted for peer-reviewed publication.
Chapter I: Influence of posttranslational modifications on protein delivery to antigen-presenting cells (not yet submitted, manuscript in preparation)
IE-1 Data from this chapter have
vaccine candidates based on Adenovirus 19a/64 exhibit broad cellular tropism and effi-
Authors: Richard Kiener, Markus Fleischmann, Christiane Schwegler, Zsolt Ruzsics, Chris- tian Thirion, Silke Schrödel, Benedikt Asbach, Ralf Wagner
Personal contribution: Richard Kiener (RK) collected parts of the data and supervised col- lection of the residual data by students. RK analyzed the data, prepared all figures and wrote the paper.
Chapter III IE-1 and pp65 (submitted)
Data from this chapter have
virus vectors efficiently transduce monocyte-derived dendritic cells and potently restimu- late T cell responses ex vivo
Authors: Richard Kiener, Markus Fleischmann, Marian Alexander Wiegand, Christiane Schwegler, Christine Kaufmann, Eva Felder, Hans-Helmut Niller, Benedikt Asbach, Ralf Wagner
Personal contribution: same as for chapter II
I. Abstract ... 7
II. Zusammenfassung ... 9
III. Introduction ... 11
III.1. Human Cytomegalovirus ... 11
III.2. Virion composition and replication ... 12
III.3. Immune responses to CMV ... 14
III.4. Clinical implications ... 17
III.5. Monitoring of CMV-specific immune responses ... 19
III.6. Current Status of CMV vaccine development ... 20
III.6.1. Modified Vaccinia Ankara (MVA) ... 22
III.6.2. Adenovirus vectors ... 23
III.6.3. Sendaivirus vectors ... 24
III.7. Objective of this thesis ... 25
IV. Results ... 27
IV.1. Influence of posttranslational modifications on protein delivery to antigen-presenting cells ... 27
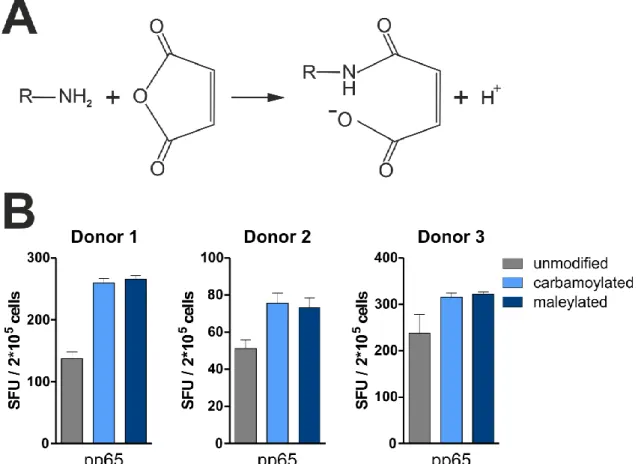
IV.1.1. Impact of protein carbamoylation on T cell restimulation ... 29
IV.1.2. Impact of negative protein charges on T cell restimulation ... 33
IV.1.3. Uptake of carbamoylated proteins into antigen-presenting cells ... 40
IV.1.4. Impact of protein carbamoylation on T cell priming in vivo ... 47
IV.2. Delivery of CMV antigens by viral vectors ... 49
IV.2.1. Delivery of IE-1 and pp65 by Adenovirus vectors ... 53
IV.2.2. Delivery of IE-1 and pp65 by Sendai virus vectors ... 61
V. Discussion ... 69
V.1. Carbamoylation enhances protein uptake into antigen-presenting cells ... 69
V.2. Ad19a/64 is an interesting alternative to Ad5 for Adenovirus-mediated gene delivery ... 76
V.3. Attenuated SeV is a promising vector system for the delivery of antigens to dendritic cells ... 79
VI. Materials and methods ... 82
VI.1. Cell culture techniques ... 82
VI.2. Baculovirus expression system ... 83
VI.2.1. Bacmid generation ... 83
VI.2.2. Transfection of insect cells with recombinant bacmids ... 84
VI.2.3. Infection of High Five cells ... 84
VI.3. Protein biochemistry techniques ... 85
VI.3.1. Bradford assay ... 85
VI.3.4. Coomassie staining ... 87
VI.3.5. Western blot analysis ... 87
VI.3.6. Silver staining ... 88
VI.3.7. Posttranslational modification ... 88
VI.3.8. Fluorescence analysis ... 89
VI.3.9. Isoelectric focusing ... 89
VI.3.10. Carbamoyl-ELISA ... 89
VI.4. Mass spectrometry analysis ... 90
VI.4.1. LC-MS analysis of amino acid composition ... 90
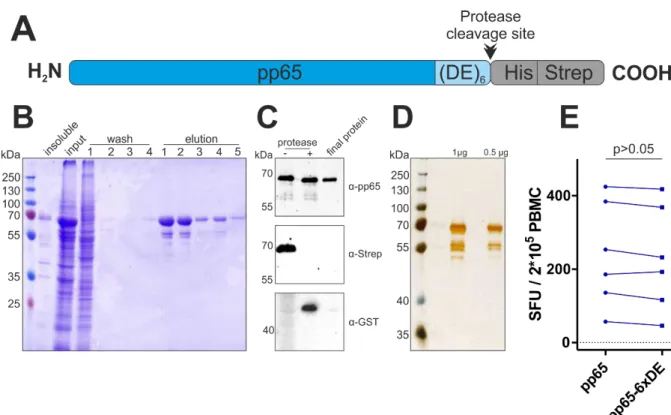
VI.4.2. MS/MS analysis of pp65-derived peptides ... 91
VI.5. Isolation, cultivation and differentiation of primary cells... 93
VI.5.1. Ethics ... 93
VI.5.2. Isolation of PBMCs ... 93
VI.5.3. ELISpot assay ... 93
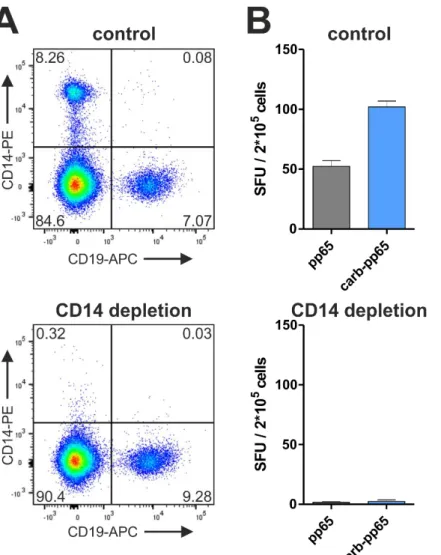
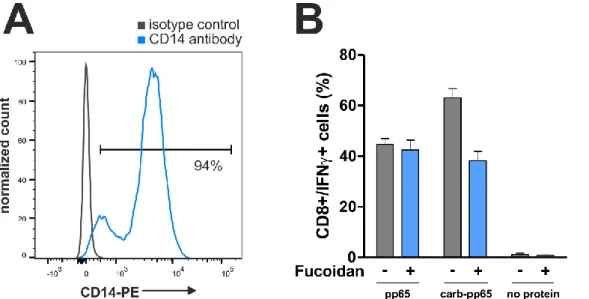
VI.5.4. MACS sorting ... 94
VI.5.5. Monocyte differentiation to moDCs or moMφs ... 94
VI.5.6. Expansion of T cell clones ... 96
VI.5.7. Mixed leukocyte reaction (MLR) ... 97
VI.6. Virological techniques ... 98
VI.6.1. Generation of recombinant MVA vectors ... 98
VI.6.1.1. In vitro recombination (IVR) ... 98
VI.6.1.2. Plaque purification ... 98
VI.6.1.3. PCR screening ... 99
VI.6.2. Generation of recombinant AdV vectors ... 100
VI.6.3. Generation of recombinant SeV vectors ... 100
VI.6.4. Viral infection ... 101
VI.6.5. Viral target cell tropism ... 101
VI.6.6. Intracellular IE-1/pp65 staining ... 101
VI.6.7. T cell restimulation by virally transduced moDCs ... 102
VI.6.8. Analysis of DC maturation ... 102
VI.6.9. AnnexinV/7AAD assay ... 103
VI.6.10. Cytokine secretion analysis ... 103
VI.6.11. Cross presentation assay ... 103
VI.7. Animal experiments ... 104
VI.10. Homology modeling and electrostatic surface potential calculation ... 106
I. Appendix ... 107
I.1. Abbreviations ... 107
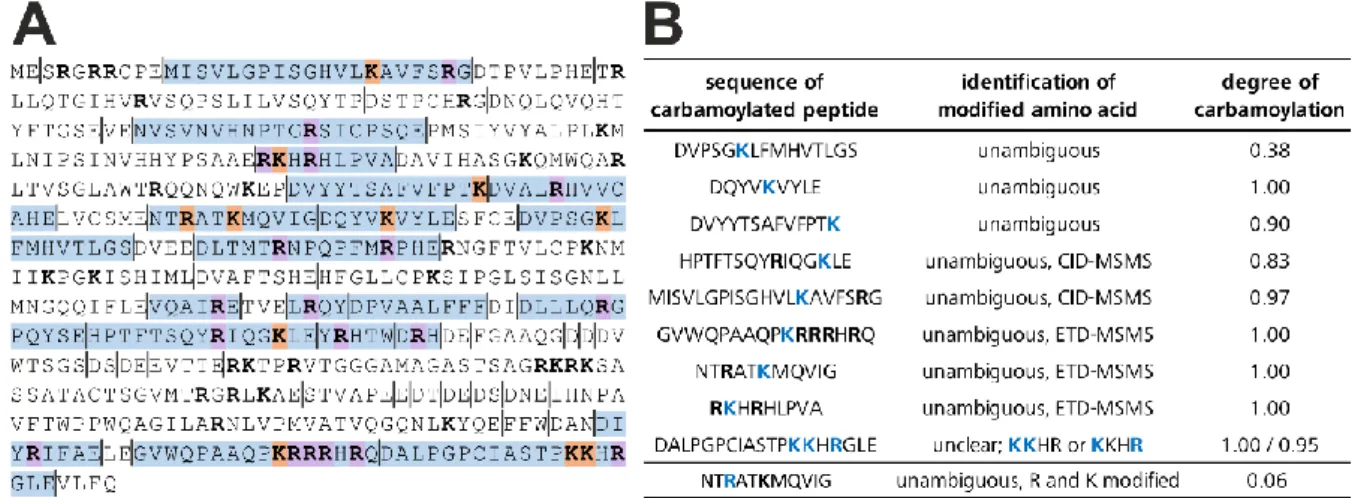
I.2. Supplementary data ... 109
I.3. References ... 115
I.4. Danksagung ... 127
7
I. Abstract
Human Cytomegalovirus (CMV) is a highly prevalent -herpesvirus that establishes life-long latency after primary infection. Congenital CMV infection is the most common viral compli- cation in newborns, causing a number of late sequelae that range from impaired hearing to mental retardation. At the same time, managing CMV reactivation during immunosup- pression remains a major hurdle in post-transplant care.
Since CMV-specific T cells are critical for controlling viral reactivation, monitoring of T cell responses is a promising strategy to identify patients at risk for symptomatic CMV disease.
T cell responses are mostly quantified after antigen-specific restimulation, which requires presentation of pathogen-derived peptides by antigen-presenting cells (APCs). Here, it was tested if protein carbamoylation, a posttranslational modification of amino groups occur- ring in vivo during renal dysfunction or inflammation, impacts the uptake of recombinant proteins into APCs and, as a result, also T cell restimulation. The CMV proteins immediate- early 1 (IE-1) and pp65, which are the main targets of T cell-mediated immunity during CMV infection, as well as the Epstein-Barr virus (EBV) protein BZLF1 were chosen as model pro- teins and recombinantly produced for addressing this question. As a result of protein car- bamoylation, antigen-specific restimulation of T cells was increased for pp65 (mean increase 39%, ranging from 6-112% for different blood donors) and BZLF1 (mean increase 80%, ranging from 5-161%), but decreased for IE-1. Mass spectrometry analysis revealed that lysine residues were almost completely modified (>90%), while arginine carbamoylation was negligible under the chosen conditions (0.3%). The removal of positive charges from amino groups as a result of carbamoylation was found to be important for the enhanced restimulation of pp65-specific T cells, since this effect could be reverted by addition of the polyanionic competitor fucoidan. In addition, protein maleylation, another posttranslational modification removing positive charges from amino groups, mediated a similar increase in the restimulation of pp65-specific T cells. T cell responses to carbamoylated pp65 were increased compared to the unmodified protein, irrespective of whether monocytes, macro- phages or dendritic cells acted as APCs. Improved restimulation of pp65-specific T cells was shown to be the result of enhanced protein uptake into APCs, which increased both MHC-I and MHC-II presentation.
In summary, protein charges appear to have a major influence on uptake into antigen- presenting cells, which could be harnessed for improving current methods for T cell im- munomonitoring and the design of novel vaccines.
In the second part of this thesis, new viral vectors delivering the T cell immunogens IE-1 and pp65 were evaluated with regard to suitability as future CMV vaccine candidates. The vec- tors were based on Adenovirus 19a/64 (Ad19a/64) or Sendai virus (SeV) and compared side- by-side to the well-established vector platforms Ad5 and Modified Vaccinia Ankara (MVA).
All vectors were characterized virologically and immunologically in a series of ex vivo assays
8 with a focus on dendritic cells (DCs), which are the main population priming T cell responses in vivo.
It was found that unlike Ad5, Ad19a/64 vectors readily transduce a broad panel of immune cells, including monocytes, T cells, NK cells and monocyte-derived dendritic cells (moDCs).
Both Ad19a/64- and MVA-transduced moDCs efficiently restimulated IE-1 or pp65-specific T cells, but MVA induced a higher amount of cytotoxicity in this cell type. Ad5 and Ad19 induced upregulation of the maturation markers CD86 and HLA-DR in moDCs whereas ex- pression of CD80 and CD83 was largely unaltered. By contrast, MVA transduction led to downregulation of all markers.
To enhance the safety of SeV vectors, a replication-deficient strain (rdSeV) that infects target cells in a non-productive manner while retaining viral gene expression was used in this the- sis. rdSeV was compared to the parental, replication-competent Sendai virus strain (rcSeV) as well as MVA. rcSeV was capable of replicating to high titers in DCs while rdSeV infected cells abortively. Due to the higher degree of attenuation, IE-1 and pp65 protein levels me- diated by rdSeV after infection of DCs were markedly reduced compared to the parental rcSeV strains, but antigen-specific restimulation of T cell clones was not negatively affected by this. Importantly, rdSeV showed reduced cytotoxic effects compared to rcSeV and MVA and was capable of mediating DC maturation as well as secretion of Interferon α and Inter- leukin 6.
Taken together, these data demonstrate that both rdSeV and Ad19a/64 have great poten-
tial as novel vector systems for the delivery of CMV immunogens.
9
II. Zusammenfassung
Das humane Zytomegalievirus (CMV) weist weltweit hohe Seroprävalenz auf und etabliert nach der Primärinfektion lebenslange Latenz. Infektionen mit CMV sind die häufigste virale Komplikation bei Neugeborenen und verursachen eine Reihe von Spätfolgen wie Schwer- hörigkeit oder geistige Behinderungen. Zusätzlich stellen CMV Reaktivierungen die bedeu- tendste pathogen-assoziierte Komplikation bei Transplantatempfängern dar.
Da T Zellen eine entscheidende Rolle bei der Kontrolle viraler Reaktivierungen einnehmen, stellt die Überwachung CMV-spezifischer T Zell Antworten eine vielversprechende Strategie zur frühzeitigen Identifikation von Patienten mit erhöhtem Risiko für symptomatische CMV Erkrankung dar. Derzeit werden pathogen-spezifische T Zellen hauptsächlich infolge von Restimulation mit entsprechenden Antigenen nachgewiesen und quantifiziert, was deren Prozessierung und Präsentation durch Antigen-Präsentierenden Zellen (APCs) voraussetzt.
In dieser Arbeit wurde untersucht, ob Carbamoylierung (eine posttranslationale Modifika- tion die auch in vivo im Zuge von Niereninsuffizienz oder Entzündungen vorkommt) die Aufnahme rekombinanter Proteine in APCs und damit die Restimulation von T Zellen beein- flusst. Hierfür wurden die CMV Proteine Immediate-early 1 (IE-1) und pp65, welche wäh- rend CMV Infektionen die stärksten T Zell Antworten hervorrufen, sowie das Epstein-Barr virus (EBV) Protein BZLF1 rekombinant hergestellt. Carbamoylierung verbesserte die Resti- mulation pp65-spezifischer T Zellen im Durchschnitt um 39%, (6-112%, je nach individuel- lem Blutspender) und BZLF1-spezifischer T Zellen um 80% (5-161%), wohingegen die IE-1 Antworten stark verringert waren. Massenspektrometrische Analysen zeigten, dass unter den gewählten Reaktionsbedingungen Lysine quantitativ modifiziert wurden (>90%), wo- hingegen Arginin-Carbamoylierung kaum nachzuweisen war (0.3%). Es zeigte sich außer- dem, dass das Entfernen positiver Ladungen infolge der Carbamoylierung notwendig für die verbesserte T Zell Restimulation ist, da sich der Effekt durch Zugabe des polyanionischen Kompetitors Fucoidan aufheben ließ. Zudem hatte Maleylierung, eine weitere posttransla- tionale Modifikation, welche das Entfernen positiver Ladungen von Aminogruppen zur Folge hat, einen ähnlichen Einfluss auf die Restimulierung pp65-spezifischer T Zellen. Die Verbesserung der T Zell Stimulation durch carbamoyliertes pp65 war unabhängig davon ob Monozyten, Makrophagen oder dendritische Zellen als APCs fungierten. Der Grund hierfür scheint eine generell erhöhte Aufnahme in Antigen-präsentierende Zellen zu sein, wobei sowohl MHC-I, als auch MHC-II Präsentation verbessert waren.
Zusammenfassend lässt sich festhalten, dass die Ladung von Proteinen die Aufnahme in APCs stark beeinflusst, was beispielsweise in die Verbesserung aktueller T Zell Diagnostika oder die Herstellung neuartiger Impfstoffe einfließen könnte.
Im zweiten Teil dieser Arbeit wurden neuartige virale Vektoren für die Verabreichung der
T Zell Immunogene IE-1 und pp65 hinsichtlich ihrer Eignung als CMV Impfstoffkandidaten
evaluiert. Die Vektoren basieren auf dem Adenovirus Subtyp 19a/64 (Ad19a/64), sowie
Sendai Virus (SeV) und wurden direkt mit den etablierten Vektoren Adenovirus Subtyp 5
10 (Ad5) und Modified Vaccinia Ankara (MVA) verglichen. Alle Vektoren wurden in einer Reihe von ex vivo Studien hinsichtlich verschiedener virologischer und immunologischer Parame- ter charakterisiert. Hierbei lag ein besonderer Fokus auf dendritischen Zellen (DCs), da diese in vivo hauptsächlich für das Priming naiver T Zellen verantwortlich sind.
Hierbei zeigte sich, dass Ad19a/64 Vektoren im Gegensatz zu Ad5 in der Lage sind, ein breites Panel an Immunzellen, bestehend aus Monozyten, T Zellen, NK Zellen und mono- cyte-derived dendritic cells (moDCs), zu transduzieren. Sowohl Ad19a/64, als auch MVA waren in der Lage, moDCs effizient zu transduzieren und die Restimulation IE-1- und pp65- spezifischer T Zell Klone zu vermitteln. Allerdings induzierte MVA in einem deutlich stärkeren Ausmaß als Ad19a/64 Zytotoxizität in DCs. Infolge der Transduktion mit Ad5 und Ad19a/64 wurden die Maturationsmarker CD86 und HLA-DR hochreguliert, wobei das Expressionsni- veau der Marker CD80 und CD83 unverändert blieb. Im Gegensatz dazu wurde die Expres- sion aller Maturationsmarker infolge der Transduktion mit MVA herabreguliert.
Um die Sicherheit SeV-basierter Vektoren zu erhöhen, wurde in dieser Arbeit ein replikati- onsdefizienter Virusstamm (rdSeV) eingesetzt, der die Expression viraler Gene in Zielzellen induziert, wobei die Bildung neuer Viruspartikel unterbleibt. rdSeV wurde sowohl mit dem parentalen, replikationskompetenten SeV Stamm (rcSeV), als auch mit MVA verglichen. Es zeigte sich, dass rcSeV effizient in humanen DCs repliziert, wohingegen Infektion mit rdSeV abortiv abläuft. Mit dem erhöhten Grad an Attenuierung von rdSeV ging, verglichen mit rcSeV, zugleich ein vermindertes Expressionsniveau von IE-1 und pp65 einher, jedoch war die Restimulation antigenspezifischer T Zellen hiervon nicht negativ beeinflusst. Es ist zudem hervorzuheben, dass rdSeV auch geringere Zytotoxizität im Vergleich mit rcSeV und MVA aufwies und die Maturation von DCs, sowie die Sezernierung von Interferon α und Interleu- kin 6 induzierte.
Insgesamt zeigen diese Daten, dass sowohl rdSeV, als auch Ad19a/64 vielversprechende
neue Vektorsysteme für die Verabreichung von CMV Immunogenen darstellen.
11
III. Introduction
III.1. Human Cytomegalovirus
The family Herpesviridae comprises more than 100 known individual virus species that are capable of infecting a wide variety of mammals, birds and reptiles
1. They share a common virion architecture that is characterized by the presence of an icosahedral nucleocapsid con- taining a linear, double-stranded DNA genome. The capsid is surrounded by an amorphous layer termed the tegument and further enveloped by a host cell-derived lipid bilayer with viral glycoproteins (Figure 1)
2. Nine herpesviruses, which are classified into three subfamilies, have humans as their primary host: α-herpesviruses (herpes-simplex virus type 1 and 2, var- icella-zoster virus) -herpesviruses (human cytomegalovirus, human herpesvirus types 6A and 6B) -herpesviruses (Epstein-Barr virus, human herpesvirus type 7, Kaposi's sar- coma-associated herpesvirus).
Figure 1 Structural features of herpesvirus particles
Herpesviruses exhibit a characteris- tic virion architecture that includes an icosahedral capsid containing a double-stranded DNA (dsDNA) ge- nome. The nucleocapsid is sur- rounded by a lipid envelope, which holds different types of glycopro- tein spikes that are involved in ad- hesion and membrane fusion. Cap- sid and envelope are separated by an unstructured layer referred to as the tegument, which contains viral and host cell-derived proteins. Fig- ure adapted from Gardner and Tortorella3.
Human cytomegalovirus (CMV, HHV-5), which owes its name to the characteristic enlarge-
y the prototype of
-herpesviruses
4. CMV is distributed globally and infects the majority of the human popula-
tion. Seroprevalence rates range, depending on the geographic region, from 30% to 100%,
but generally increase with age
5 7. Like all herpesviruses, CMV establishes life-long latency
with periodic reactivations after primary infection
8. Horizontal transmission can occur
through close contact with persons who excrete the virus in their body fluids, for example
by kissing, sexual contact or breastfeeding. Blood transfusion, solid-organ transplantation
(SOT) or hematopoietic stem cell transplantation (HSCT) constitute additional routes of CMV
transmission
9. At the same time, vertical transmission from mother to child can occur pre-
or perinatally as a result of primary infection or viral reactivation during pregnancy
10. Given
that persistently infected individuals are not protected from reinfection despite virus-specific
cellular and humoral immunity, superinfection with an additional CMV strain can also in-
crease the risk of mother-to-child transmission
11,12.
12
III.2. Virion composition and replication
CMV, which is among the most complex human-pathogenic viruses known, displays a virion architecture that is characteristic of all herpesviruses (Figure 1). With approximately 235 kilobase pairs (kb), CMV has the largest genome of all herpesviruses known to cause disease in humans
13. The exact number of genes encoded by CMV is still a matter of debate and varies, depending on the annotation criteria, strongly from about 150 to more than 230
1416
. Next to an undefined number of protein-coding genes, CMV also encodes several long noncoding- and micro-RNAs
1,17. The genome is flanked by terminal repeat (TR) sequences and divided by internal repeats (IR) into two individual genomic regions that are referred to as unique long (UL, upstream of IRs) and unique short (US, downstream of IRs; Figure 2).
Accordingly, CMV genes are named by a prefix indicating the unique region in which they are found and numbered sequentially
18,19. The genome is enclosed by an icosahedral protein capsid made up of 162 capsomers. It is formed by 4 capsid proteins termed major capsid protein (MCP, UL86), triplex 1 (TRI1, UL46), triplex 2 (TRI2, UL85) and smallest capsid pro- tein (SCP, UL48A)
20.
Figure 2 Schematic representation of the Cytomegalovirus genome
The genome of CMV is flanked by repeat sequences referred to as terminal repeat long (TRL) and terminal repeat short (TRS). Additional repeat sequences, termed internal repeat long (IRL) and internal repeat short (IRS), divide the genome into two individual sections which contain protein-coding genes: unique long (UL) and unique short (US).
The capsid is surrounded by the viral tegument, an unstructured layer that contains a high number of viral proteins. In fact, more than half of the 71 viral proteins that are found within CMV particles are located in the tegument, along with numerous host cell-derived proteins
21. During viral replication, tegument proteins are involved in a number of key steps such as capsid delivery to the nucleus (UL47 and UL48
21), gene regulation (UL82
22) and assembly of newly generated virions (UL99
23), yet the function of many tegument compo- nents remains elusive. Evasion of innate and adaptive immune responses, which is critical for viral replication, is also carried out in part by tegument proteins. For instance, the phos- phoprotein pp65 (UL83), which is the most abundant protein both in the tegument as well as in the entire virion
15, protects infected cells from innate immunity by inhibiting natural killer (NK) cell cytotoxicity through interaction with the NKp30 activating receptor
24. In ad- dition, pp65 also counteracts adaptive immune responses by mediating the accumulation of HLA class II molecules in lysosomes and inducing the degradation of the HLA-DR α-chain in this compartment
25.
Capsid and tegument are encased by a host-derived lipid envelope containing various gly- coprotein complexes (GCs) that are necessary for entry into different cell types (Figure 3).
These complexes comprise oligomers of gB (GC I; encoded by UL55), the gM/gN dimer (GC
13 II; UL100/ UL73), the gH/gL/gO trimer (GCIII; UL75/UL115/UL74) and the pentameric com- plex (PC) consisting of gH/gL and UL128/UL130/UL131a. The core fusion machinery consists of the proteins gB, gH and gL, which are conserved throughout the herpesvirus family
26. Entry into fibroblasts takes place by macropinocytosis, followed by the fusion of viral and cellular membranes. This process is mediated by GC I and GC III and requires no changes in pH
27,28. By contrast, entry into epithelial, endothelial, dendritic cells and monocytes requires virion uptake via endocytosis or macropinocytosis and a fusion event that relies on vesicle acidification. This entry pathway is mediated by GC I, GC III and PC
29 33. The function of GC II in these processes is still not fully understood, but it is presumed to induce initial tethering of virus particles to target cells through interaction with heparan sulfate proteoglycans
34.
Figure 3 Quaternary structure and topology of glycoprotein complexes Glycoproteins (gp) on CMV virions are present in several glycoprotein complexes (GCs). These complexes include GC I (gB, which consists of gp58 and gp116), GC II (gM and gN), GC III (gH, gO, gL) and the pentameric complex (PC, gH/gL and UL128/130/131a). Orange lines indicate subunits that are held together by disul- fide bonds. Figure adapted from Gardner and Tortorella3.
Upon entry, the capsid is released into the host cell cytoplasm and transported along mi- crotubules to the nuclear membrane, where viral DNA is inserted through nuclear pores
35. Expression of CMV genes occurs in three sequential phases termed immediate-early (IE), early (E) and late (L). Immediate-early genes are the first to be transcribed and their proteins mediate the transcription of early gene products which are, among others, responsible for the replication of viral DNA
36,37. In turn, the presence of early-phase proteins is a prerequisite for transition into the late phase, where structural components for the assembly of progeny virions are mainly synthesized
38.
Expression of IE genes occurs immediately upon nuclear entry of CMV DNA and does not
require de novo synthesis of viral proteins. IE genes in the UL region comprise the IE-A
(major immediate early) and IE-B locus as well as UL115-119. Additional IE genes located in
the US region include US3 and the TRS1/IRS1 genes
36. From the major immediate-early lo-
cus, multiple proteins are being produced by alternative splicing, with the 72 kDa IE-1
(UL123) and the 86 kDa IE-2 (UL122) being the most abundant and important ones
39. IE-2
is the principal activator of early genes and its presence is indispensable for viral replica-
tion
40. IE-1 is also capable of mediating early gene expression, but under certain in vitro
conditions like infection at high multiplicities of infection (MOIs), viral replication can also
be completed in the absence of this protein
41. In addition to viral genes, IE proteins also
modulate a number of host genes
36. IE-1 and IE-2 further contribute to viral immune evasion
by various mechanisms, for instance by blocking apoptosis
42or dampening cytokine release
from infected cells by interfering with JAK- -signalling
43 45. Moreover, IE-1
14 also antagonizes host immunity by promoting the disassembly of PML nuclear bodies, which are key mediators of innate immune responses within the cell nucleus
46 48.
Early genes mostly function in one of two ways: One subset of gene products is directly involved in viral DNA synthesis, cleavage and packing of the viral genome. The other subset creates a cellular environment that is ideal for viral replication, for example by modulating factors that are involved in control of cellular DNA synthesis or by contributing to the eva- sion of immune responses
37. Viral DNA synthesis occurs in the host cell nucleus from a ge- nomic region termed oriLyt and requires, next to some cellular factors, a variety of viral proteins including the helicase-primase complex (UL105, UL70 and UL102
49), the viral DNA polymerase (UL54)
50as well as its accessory protein (UL44)
51and the single-stranded DNA binding protein (UL57)
52. Replication of the viral genome is an essential activation event for the expression of late genes which are required for virus assembly and egress
38. Capsid assembly and DNA packaging occur in the nucleus, followed by translocation of DNA-con- taining capsids into the cytoplasm, which is mediated by the virally encoded nuclear egress complex (NEC, UL50 and UL53)
53,54. During nuclear egress, capsids obtain a primary enve- lope derived from the inner nuclear membrane, which is subsequently lost by fusion with the outer nuclear membrane
55,56. Further maturation steps, during which capsids obtain their final tegument layer, then take place in the cytoplasm
57. CMV virions obtain their sec- ondary envelope, including viral glycoproteins, by budding into vesicles of the trans-Golgi network. Finally, mature virions are released by fusion of the vesicle membrane with the plasma membrane of the host cell
58.
In some cell types, transcription of IE genes is suppressed, a scenario in which CMV is ca- pable of switching from lytic replication to a latent phase
59. During latency, no progeny virus is produced and only a small set of latency-associated genes is transcribed
60. Cells from the myeloid lineage like CD34
+progenitor cells, monocyte precursors and monocytes have been proposed as a site of latency for CMV
61 64. Reactivation of lytic replication presumably occurs as a result of terminal monocyte differentiation, for example to dendritic cells
65,66.
III.3. Immune responses to CMV
Upon infection, CMV triggers a multitude of innate and adaptive host immune responses.
Although the virus cannot be eliminated entirely as soon as latent reservoirs are established, CMV reactivation events are efficiently confined in immunocompetent individuals, thereby inhibiting systemic virus spread and symptomatic CMV disease. This life-long interaction with the immune system leaves a profound imprint on various effector cell populations, particularly on T cells, B cells and NK cells.
As a first line of defense, CMV is subject to innate sensing by Toll-like receptors (TLRs) and
other pattern-recognition receptors
13. This is exemplified by the activation of TLR3 and TLR9
during infection with murine cytomegalovirus (MCMV)
67,68, or the interaction of gB/gH from
15 human cytomegalovirus with TLR2
69,70. Toll-like receptor engagement leads to the produc- tion of inflammatory cytokines, ultimately resulting in the activation of effector cell subsets, such as NK cells.
An important role for NK cells in controlling CMV infection has been firmly established from studies demonstrating their involvement in the clearance of experimental MCMV infec- tion
71,72. Moreover, the adoptive transfer of NK cells can provide protection from MCMV infection in mice
73, and in humans, CMV infection can occasionally be controlled by NK cells, even in the absence of additional T cell responses
74. Natural killer cells are a highly diverse group of lymphocytes that are capable of eliminating pathogen-infected or cancer- ous cells through the secretion of cytolytic effector molecules
38. They are divided into vari- ous subsets, based on the expression of characteristic combinations of activating and inhib- itory cell surface receptors. NK cell activation is regulated by a fine-tuned integration of signals from those invariant, germline-encoded receptors
75. It was recently demonstrated that infection with Cytomegalovirus induces the clonal expansion of NK cells expressing the activating receptor NKG2C
76. While NKG2C
+cells normally make up only a minority of NK cells in peripheral blood, their frequency was found to be markedly increased upon acute CMV infection
77. Such expansion of NKG2C
+cells has also been described for other viral infections such as HIV and HBV, but was only observed in CMV seropositive individuals
78. s required for secondary expansion of this NK subset in response to other viral infections. Nevertheless, the initial activation of NKG2C
+NK cells is likely not a result of direct recognition of CMV, but rather by differences in the expression of MHC-I molecules on infected cells, especially upregulation of HLA-E
79. Along with selective expansion, NKG2C
+cells from CMV seropositive donors were also found to produce more IFN- upon stimulation, compared to the identical NK subset from CMV ser- onegative donors
80. These findings, which imply immune memory-like properties, are fur- ther underlined by profound epigenetic alterations in NKG2C
+NK cells as a result of CMV infection
81. Such results challenge the traditional view of innate immune cells as being short- lived and incapable of retaining any form of memory and blur the lines between innate and adaptive immunity
82.
Next to NK cell mediated immunity, CMV infection also triggers antibody responses against
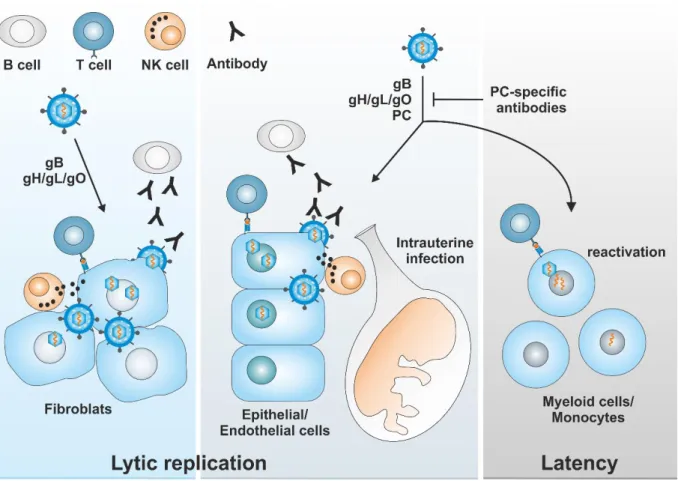
the various glycoprotein complexes that are located on the virion surface (Figure 3)
83. The
contribution of antibody responses to protection from Cytomegalovirus infection is contro-
versial, in part due to the fact that seropositive individuals can get superinfected with an-
other CMV strain even despite detectable antibody responses. However, there is substantial
evidence supporting a role for humoral immunity in restricting viral dissemination and lim-
iting the severity of CMV disease
13,84,85. During natural CMV infection, detectable antibody
responses are mostly directed against gB, gH/gL and Pentameric complex (PC)
86,87. Of those,
PC-specific antibody responses appear to have the highest neutralizing capacity and vac-
cines that are based on this complex elicit strong and broadly neutralizing responses in
different animal models
88 91. PC-specific antibodies are also 100- to 1000-fold more potent
16 in inhibiting epithelial and endothelial cell infection compared with those targeting gB or gH/gL/gO
92. Consequently, such antibody responses may be superior in preventing the in- fection of placental cytotrophoblasts, which adopt an endothelial phenotype during gesta- tion (Figure 4)
83. Although many molecular determinants of intrauterine CMV infection are still unclear, infection of this cell type is thought to be a critical event during CMV transmis- sion to fetuses
93,94. This is supported by studies that found a reduced risk of fetal transmis- sion when primary CMV infection during pregnancy was concomitant with early develop- ment of PC-specific antibodies
95,96. As a result, the PC is a central component of many cur- rent vaccine concepts that aim at the induction of antibody responses for the prevention of mother-to-child transmission
97.
Despite an undisputable contribution of NK- and B cells, T cell-mediated immunity is still considered the predominant mechanism by which latent CMV infection is controlled in vivo
13. CMV infection induces profound changes in the T cell memory compartment and an extraordinarily large proportion of T cells is often dedicated solely to this pathogen. Domi- nant responses to single epitopes regularly reach 5-10% of total CD8
+T cells in peripheral blood and up to 30% of the overall cytotoxic T cell (CTL) responses are sometimes CMV- specific in healthy adults
98,99. However, there is considerable variation in magnitude and breadth of T cell responses between individuals, a phenomenon that is still not fully under- stood
100. Typically, T cell responses to CMV tend to increase with age, display an effector memory (T
EM) phenotype and lack markers of T cell exhaustion
99,101,102. The gradual expan- sion and long-term maintenance of CMV-specific T cell populations after initial infection is
ally defined and was originally introduced in the context of MCMV infection
103. It is remarkable that large numbers of CMV-specific T cells are sustained over many years without diminished effector functions, given that in other chronic viral infections such as HIV, HBV or HCV, virus-specific T cells typically decrease in number and show signs of exhaustion over time
104,105. It has been hypothesized that this is due to differences in the antigenic burden, since the aforementioned viruses typically replicate continuously and to high titers in vivo.
This results in constant stimulation of virus-specific T cells, a condition that favors functional exhaustion over time. By contrast, CMV reactivation from latency seems to occur sporadi- cally and on a smaller scale, therefore avoiding permanent restimulation of T cells
106,107. However, it is still unclear if the extraordinarily high number of CMV-specific T cells in some individuals is a requirement for protection or simply the result of gradual, life-long expan- sion of an initial pool of effector cells that is driven by periodic reactivation events
108. Due to its profound and long-lasting impact on the immune system, CMV is also suspected of accelerating immune senescence, but it is currently not clear if this has clinical implica- tions
109,110.
Although many questions about the nature of the CMV-specific T cell pool are still un-
addressed, their potency in controlling CMV infection is well established. For instance, an-
tibody-mediated depletion of CD8
+T cells in rhesus monkeys leads to reactivation of
17 rhCMV
111. Depletion of lymphocytes in mice also results in reactivation of MCMV replica- tion, which can be suppressed by adoptive transfer of T cells
112,113. Similarly, CMV-specific T cell responses and subsequent control of viral replication, could be restored in immunocom- promised patients through adoptive T cell transfer
114 116. T cell responses are directed against a high number of CMV proteins: Using overlapping 15-mer peptides comprising 213 CMV genes for the stimulation of T cells from 33 seropositive adults, Sylwester et al. found that a total of 151 open-reading frames (70%) were immunogenic
98. Most individuals rec- ognize 5-10 proteins in each T cell subset, with IE-1, IE-2 and pp65 generally inducing the strongest CTL responses and gB, pp65, UL16 and TRL14 dominating the CD4
+T cell com- partment
13,108.
Figure 4 Immune responses to CMV
CMV is capable of infecting a wide variety of cells ranging from fibroblasts to epithelial cells, endothelial cells and myeloid cells (e.g. monocytes and precursors thereof). Fibroblast infection is mediated by the glycoprotein complexes gB (GC I) and gH/gL/gO (GC III), while for the infection of the residual cell types, the pentameric complex (PC) is required in addition to GC I and GC III. Lytic replication is confined (among others) by T cell, B cell and NK cell mediated antibody responses. Antibodies that are directed against the pentameric complex are particularly potent in inhibiting endothelial/epithelial cell infection and might therefore be superior in pre- venting intrauterine infection. During latency, which is established in certain cells from the myeloid lineage, only a small set of latency-associated genes is expressed. Viral reactivation from latently infected cells (for example as a result of monocyte differentiation) is controlled predominantly by T cell mediated immunity.
III.4. Clinical implications
In healthy individuals, primary infection is mostly asymptomatic or merely accompanied by
mild symptoms resembling those of an infectious mononucleosis
117. However, CMV causes
18 considerable morbidity and even life-threatening complications in immunocompromised in- dividuals such as transplant recipients and AIDS patients
118 120. Cytomegalovirus particles can infect a remarkably broad range of cells and spread to virtually every organ within its host
121. As a result, CMV disease can result in a variety of clinical manifestations such as colitis, pneumonitis, encephalitis, hepatitis and retinitis
122,123.
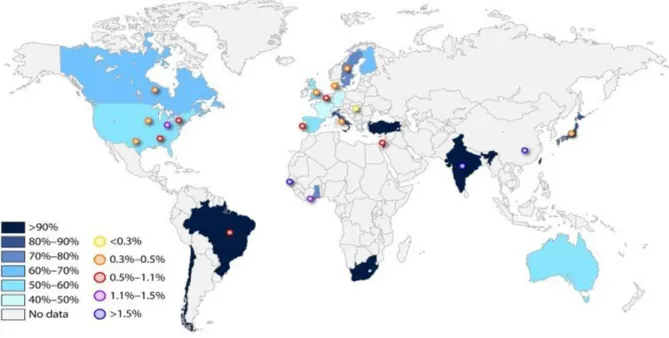
Human cytomegalovirus also represents one of the most common congenital infections worldwide with an estimated prevalence of 0.64% at birth and is a leading cause of disa- bility in children (Figure 5)
124,125. The risk for intrauterine transmission is highest (30-35%) if a seronegative mother becomes infected with CMV during pregnancy
125. By contrast, viral reactivation in a seropositive mother less frequently results in fetal infection (1.2-1.4%)
7,126. Neonatal symptoms of congenital CMV infection include microcephaly, hepatosplenomeg- aly, petechiae, jaundice, chorioretinitis, thrombocytopenia and anemia
127. The mortality rate among infants with symptomatic CMV disease at birth is 10 30% and only about 10% fully recover, while the remaining children will have long-term sequelae ranging from sensori- neural hearing loss and delayed neural development to mental retardation
128,129. However, even children with congenital CMV infection that show no symptoms at birth may develop late sequelae, albeit with lower frequency (8-15%)
130,131. Early infection of the fetus, partic- ularly in the first trimester, is generally associated with an increased likelihood of severe complications
132.
Figure 5 - Global CMV seroprevalence levels and incidence of congenital CMV infection
Cytomegalovirus seroprevalence rates among women of reproductive age (reproduced from Manicklal et al.124) are displayed in different shades of color, and congenital CMV birth prevalence rates are shown by the circles. Figure from Emery and Lazzarotto132.
CMV is considered an opportunistic pathogen, mostly causing severe disease as a result of
inadequate immune responses. Besides congenital infections, where the virus often encoun-
ters an immune system that is not fully developed yet, this is also the case during innate or
19 acquired immunodeficiency. Accordingly, an increased risk for CMV disease has been de- scribed in numerous innate immunodeficiency syndromes like Fanconi anemia, severe com- bined immunodeficiency (SCID) or common variable immunodeficiency (CVID)
133 135. Ac- quired immunodeficiency, mainly due to HIV infection, also predisposes for symptomatic CMV disease. The broad application of combined antiretroviral therapy (ART) has reduced the incidence of CMV disease from being the most significant opportunistic infection to a rarity during treatment
122,136,137. However, given that a high proportion of HIV positive indi- viduals is still without access to ART, CMV remains a significant co-morbidity in patients suffering from AIDS.
Because the number of solid-organ and haematopoietic stem cell transplantations has been increasing in recent years, transplant recipients are another steadily growing group with a high incidence of CMV disease. New potent immunosuppressive agents contribute to de- creasing the incidence of graft rejection, but at the same time, patients are left highly sus- ceptible to opportunistic infections
138. Although various pathogens frequently cause disease in transplant recipients (e.g. EBV, Adenoviruses, mycobacteria or fungi), CMV is still the most common infection
139 142. The risk for severe complications is generally greatest if a CMV seronegative donor receives a graft from a seropositive donor (D+/R-)
143. However, a multitude of additional factors such as the age of the transplant recipient, the state of im- munosuppression, genetic predispositions or the type of organ graft influence the clinical outcome as well: For example, lung, small intestine and pancreas transplant recipients are at higher risk for severe complications than kidney or liver transplant recipients
118,144. Antiviral agents such as Ganciclovir, Valganciclovir, Foscarnet and Cidofovir are available for the treatment of CMV disease and capable of reducing the incidence of CMV reactivation after transplantation
145 147. However, renal toxicity and interactions with other drugs, as well as the emergence of resistant virus strains, limit their therapeutic potential
148 150. Thus, to avoid unnecessary administration of antivirals, preemptive therapy is often applied instead of universal prophylaxis for the management of CMV reactivation after transplantation
151,152. As part of this strategy, antiviral therapy is only initiated if a predefined threshold level of viral DNA copy number in blood is surpassed, because viremia usually precedes symptomatic CMV disease
136,153. Although preemptive therapy is useful for reducing drug-related toxici- ties, there is still controversy about whether it is preferable to universal prophylaxis, espe- cially in high risk constellations (D+/R-)
152,154. Next to small molecule drugs interfering with viral replication, CMV reactivation can also be controlled by adjusting the level of immuno- suppression or through adoptive transfer of virus-specific T cells, but early identification of patients at risk for viral relapse is critical for the success of all these interventions
155 157.
III.5. Monitoring of CMV-specific immune responses
Due to the key role of T cell mediated immune responses in controlling CMV replication,
there is a strong correlation between loss of CMV-specific T cells and viral reactivation. As
20 early as 1991, Reusser et al. reported that in a cohort of bone marrow transplant recipients, none of the patients with detectable CMV-specific T cells developed CMV disease. At the same time, more than half of patients lacking CMV-specific T cell responses died of CMV pneumonia
158. Since then, a plethora of studies was published highlighting the diagnostic value of virus-specific T cells for the prediction of CMV reactivation
159 162. For example, more recent work from Espigado et al. showed that after hematopoietic stem cell transplantation, early reconstitution of CMV-specific T cell responses ( 6 weeks), is associated with less incidence of CMV replication, reduced viral loads and better overall survival, compared to patients with delayed immune reconstitution
163. Collectively, these results indicate that monitoring of virus-specific T cells is a promising approach for the early identification of patients that are at risk for CMV disease (Figure 6).
Figure 6 Immune monitoring for the identification of patients at risk for CMV disease
Following transplantation, the quantity or function of T cells may decline as a result of drug-induced immunosuppression. Loss of CMV-specific T cell responses can result in re- activation of lytic CMV replication, concomi- tant with viremia and symptomatic disease.
Quantification of CMV-specific T cells could help to identify patients with an elevated risk for CMV disease prior to viremia (which is the current criterion for initiating the administra- tion of antivirals during preemptive therapy).
Various methods are currently available for assessing pathogen-specific T-cell immunity
164. Such techniques are either based on direct detection of cells, for example after staining with MHC-multimers
165, or the quantification of activation markers such as Interferon after stimulation with pathogen-derived antigens
166. These markers can then be detected by intracellular cytokine staining (ICS), followed by flow cytometry analysis, or via Enzyme- linked immunosorbent (ELISA) or Enzyme-linked immunospot (ELISpot) methods.
Next to the detection method, various types of antigens are also available for stimulation, including viral lysates, peptide pools and recombinant proteins. IE-1 and pp65 are the pre- ferred antigens for CMV in most assays, since they are the main targets of CD8
+T cells
13,167. Currently, there is no consensus on the ideal method for monitoring of CMV-specific T cells and proposed threshold levels still lack extensive validation
156. Nevertheless, there is accu- mulating evidence that in the future, monitoring T cell immunity could become a corner- stone in the management of Cytomegalovirus in transplant recipients.
III.6. Current Status of CMV vaccine development
In 2000, a committee from the US national institutes of health tasked with prioritizing ef-
forts for the development of novel vaccines assigned the highest level of priority to the
21 implementation of a CMV vaccine
168. Taking the impact of CMV on morbidity and mortality into consideration, the committee estimated that if a vaccine with 100% efficacy was ad- ministered to the entire population, a total of 70.000 quality-adjusted life years (QALY) could be gained per year in the United States. In addition, the annual health care costs saved would be an estimated 4 billion per year in the US alone (assuming that the vaccine would cost 50$ per course). However, although the first vaccine efforts date back to the 1970s and a multitude of candidates has been developed since, a protective or therapeutic CMV vaccine has still not been licensed (Figure 7)
169. An efficacious Cytomegalovirus vaccine is urgently needed, given that treatment options with antiviral agents are limited due to side effects and the emergence of resistant virus strains
170 172. In addition, there is currently no therapy available for inhibiting intrauterine infection during pregnancy, although at least some concepts are undergoing clinical trials
132,173.
Figure 7 History of CMV vaccine development
Summary of selected candidate vaccines that were evaluated in preclinical and clinical trials. Glycoprotein B (gB), pp65 and IE1 were mainly tested as potential targets, delivered by various platforms including the atten- uated CMV Towne isolate174, recombinant viral vectors encoding full-length antigens and epitopes175,176, DNA177, dense body (DB)178 and subunit179 vaccines. BAC: Bacterial artificial chromosome; CTL: Cytotoxic T- lymphocyte; MVA: Modified vaccinia Ankara. Figure from Dasari et al.180
Of the numerous candidate vaccines that were developed over the last decades, only few
made it past the early stages of clinical trials
136. However, modest efficacies have previously
been reported in phase II studies for both prophylactic as well as therapeutic approaches
22 (e.g. 50% efficacy for gB protein with adjuvant MF59)
181,182. While these results are encour- aging and imply that the development of a CMV vaccine is feasible, it also underlines the necessity to explore novel, alternative vaccine concepts.
Since clear immunological correlates of protection from CMV infection have not been de- fined yet, vaccine candidates should ideally induce both humoral and cell-mediated immune responses
180,183. As described above (section III.2), several different glycoproteins are ex- pressed on the virion surface, all of which are targets for humoral immunity. Until recently, subunit vaccines designed for the induction of protective antibody responses mostly fo- cused on the fusion protein gB. The rediscovery of the pentameric complex in 2005 as a crucial component for the infection of epithelial and endothelial cells revealed a new target for such vaccine concepts
184. Indeed, inclusion of the pentameric complex into subunit vac- cines or attenuated virus led to promising results in animal models
185,186.
At the same time, cell-mediated immunity plays a crucial role in controlling viral latency and limiting virus spread. T cell responses are directed against a variety of viral antigens, with the tegument protein pp65 and the transcriptional regulator IE-1 representing major tar- gets
98,187. Interestingly, expression of CMV antigens is frequently detected in glioblastoma cells
188,189, and as a result, therapeutic vaccination concepts that aim at expanding CMV- specific T cells for enhancing antitumor immunity are currently being tested in clinical stud- ies
190.
Viral vectors are a favored tool for the delivery of heterologous antigens, in part due to their capability to efficiently prime T cell responses during vaccination
191. Several such vectors, like the poxvirus strain Modified Vaccinia Ankara (MVA) are currently being evaluated as therapeutic vaccine candidates in clinical trials, although their efficacy has yet to be demon- strated
192,193. However, repeated administration of an antigen by a given vector is impeded by the development of immunity to its backbone, which can be avoided by heterologous prime/boost immunizations. Hence, novel vectors should still be developed and assessed for their capacity to deliver CMV immunogens. Three different viral vector classes that were used in this work (MVA, AdV and SeV) are introduced in the following sections.
III.6.1. Modified Vaccinia Ankara (MVA)
Poxviruses containing foreign genes are a well-established platform for the development of
novel vaccines and therapeutics in biomedical research. Advantages of poxvirus vectors in-
clude large packaging capacity for heterologous DNA, exclusive replication in the cytoplasm
(minimizing the risk of genomic integration) and lack of persistence in the host, as well as
high immunogenicity
194,195. Most poxvirus vectors are based on the strain Vaccinia, which
was originally used for the eradication of smallpox
196. Vaccinia was long presumed to be
derived from a strain of cowpox, but more recent analysis indicates that it likely originated
from a strain of rodent or equine origin
197. Although vaccinia exhibits strongly reduced vir-
ulence in humans, there are multiple well-documented cases of vaccination complications
23 as well as laboratory infections
198,199. Hence, several highly attenuated, Vaccinia-derived vi- rus strains were generated for usage as immunogen delivery vectors, including New York Vaccinia Virus (NYVAC)
200and Modified Vaccinia Ankara (MVA)
201. MVA was generated from the vaccinia virus strain Ankara, which was used at the vaccine institute in Ankara for smallpox vaccine production
194. Serial passaging of this strain on chicken embryo fibroblast (CEF) cells led to the loss of approximately 15% of the viral genome over more than 500 passages
202,203. The resulting MVA strain displays enhanced attenuation, as illustrated by its inability to replicate in human cell lines
204. MVA is currently licensed as a vaccine against smallpox. Moreover, it is one of the most broadly used viral vectors for the delivery of anti- gens from various pathogens.
III.6.2. Adenovirus vectors
Human adenoviruses (AdVs) comprise a large family (>70 serotype) of non-enveloped, dou- ble-stranded DNA viruses that are subdivided into seven species termed A-G
1,205,206. Depend- ing on the serotype, AdV infection can affect the respiratory, gastrointestinal or urinary tract as well as the eye, occasionally causing severe disease. Nonetheless, natural infections with these ubiquitous viruses are mostly asymptomatic or merely accompanied by mild symp- toms
207. Recombinant, replication-defective adenoviruses are extensively utilized as vectors for vaccination, cancer treatment or the delivery of therapeutic genes. Reasons for the pop- ularity of AdVs as vaccine vectors include high packing capacity and immunogenicity, com- bined with an excellent safety profile and the capability to infect both dividing and non- dividing cells
208 211. Simple and inexpensive methods for vector construction and purification of high titer viral stocks from cell culture further contribute to making the AdV vector plat- form versatile in use.
Historically, most studies on basic aspects of Adenovirus biology were carried out using AdV type 5 (Ad5, a member of subgroup C), and as a consequence, recombinant vectors were almost exclusively based on Ad5 for many years
212. However, broad usage of these vectors is limited by preexisting immunity to Ad5 in humans with the presence of neutralizing anti- bodies (NAbs) reaching up to 90% in some regions
213. Efficient transduction by Ad5 is also confined to cells expressing the Coxsackie virus and Adenovirus receptor (CAR)
214. Direct binding to erythrocytes, liver sequestration of virions and hepatotoxicity after intravenous administration constitute additional disadvantages of Ad5-based vectors counteracting broad clinical application
215 217.
In order to exploit the natural diversity of Adenoviruses and to overcome the limitations of
Ad5-based vectors, an increasing number of AdVs from different subgroups have been vec-
torized in recent years
218. Vector alternatives like Ad6 (NAb frequency 68%
213), Ad26 (NAb
frequency 43-68%
219) or Ad35 (NAb frequency 5-18%
219) were demonstrated to be
immunogenic and well tolerated in animal models and humans
220 223. Beyond that, chim-
panzee Adenoviruses (chAdVs) like chAd3 and chAd63 are also emerging as a new vector
24 class, although preexisting immunity in humans (up to 33% NAb frequency for chAd63
224) has been reported as well
225 227. While the aforementioned AdV-based vaccine candidates mostly gave promising results in clinical trials, it has also become evident that repeated administration of the same vector is hampered by the induction of neutralizing antibod- ies
228. This underlines that novel AdV vectors should still be established to meet an increas- ing demand for safe and efficacious delivery systems in gene therapy and vaccination
229. Previously, an E1/E3-deleted gene therapy vector based on Adenovirus 19a (recently re- named to Ad64
230, NAb frequency 16-19%
231,232), a member of subgroup D that causes epidemic keratoconjunctivitis in humans, has been described
233,234. AdVs from this subgroup display a particularly broad host cell tropism since they bind to ubiquitously expressed sialic acids rather than CAR and might therefore be a promising alternative to Ad5-based vec- tors
235,236.
III.6.3. Sendaivirus vectors
Sendai virus (SeV) is a non-segmented, negative strand RNA virus that belongs to the family
Paramyxoviridae and causes respiratory infections in mice
237. A number of advantageous
features have led to broad usage of SeV as a viral vector including exclusive replication in
the host cell cytoplasm
238, efficient transduction of both dividing and non-dividing cells
239,
broad target cell tropism
240,241and replication to high titers in cell culture
242. Importantly, it
is also considered non-pathogenic in humans
243,244. Sendai virus is currently tested as a Jen-
nerian vaccine for human parainfluenza virus and as a viral vector for the delivery of human
respiratory syncytial virus antigens
245–247. In appreciation of its many favorable characteris-
tics, SeV is also emerging as a vector for the delivery of immunogens from unrelated path-
ogens such as HIV-1 Gag
222,248.
25
III.7. Objective of this thesis
The quantification of virus-specific T cell responses is a promising method for assessing cell- mediated immunity to CMV in general and to predict viral reactivation from latency. Most methods for the identification of such T cells are based on the detection of activation mark- ers like upon antigen recognition via the T cell receptor complex (TCR). A prerequisite for TCR recognition of pathogen-derived peptides is their presentation by antigen-present- ing cells (APCs) and accordingly, the efficiency with which antigens are delivered to these cells has a major impact on assay sensitivity (Figure 8). Thus, one main focus of this work was to increase the uptake of proteins into APCs to improve currently available methods for the measurement of cell-mediated immunity to CMV.
Figure 8 Protein uptake and pro- cessing by antigen-presenting cells Proteins are taken up from the extracellular space (1), degraded in endolysosomes (2), loaded onto MHC-II molecules and trans- ported to the cell surface where they can interact with CD4+ T cells (3). Alternatively, in a process termed cross-presentation, pro- teins escape endosomes and enter the cyto- plasm, followed by proteasomal degrada- tion (4). Peptides are then transferred to the endoplasmic reticulum, loaded onto MHC-I molecules and transported to the cell sur- face where they are recognized by antigen- specific CD8+ T cells (5). Enhanced protein uptake might increase the overall amount of MHC presentation and, as a conse- quence, also T cell stimulation.
Previously, it was reported by Barabas et al. that storage of the Epstein-Barr virus (EBV) protein BZLF1 in high molar urea solutions prior to stimulation of immune cells enhances the restimulation of antigen-specific T cells
249. Since this is a simple and inexpensive method for increasing T cell reactivation rates, the molecular mechanism underlying this phenome- non should be elucidated in order to evaluate whether this technique can be applied to a broader panel of antigens, comprising the CMV proteins IE-1 and pp65 BZLF1.
As storage in urea induces protein carbamoylation, the main hypothesis to be tested here was that this posttranslational modification alters antigen uptake and T cell restimulation rates.
Beyond ex vivo applications, CMV proteins that are modified for enhanced uptake into APCs
might also be used for the priming of adaptive immune responses during vaccination. Fur-
ther, in order to influence magnitude and quality of the induced immune responses, a com-
bination of various delivery methods in heterologous prime/boost immunizations might be
required. Of the many conceivable delivery systems, live attenuated viral vectors are partic-
ularly interesting tools due to their inherent immunogenicity. Hence, another main focus of
this work was the generation and preclinical characterization of novel virus vectors deliver-
26 ing CMV antigens. Two adenoviruses strains (Ad5 and Ad19a/64), the poxvirus strain Mod- ified Vaccinia Ankara (MVA) as well as Sendai virus (SeV, a murine RNA virus) were chosen and recombinants expressing IE-1 or pp65 were generated. Since immunological infor- mation on SeV and Ad19a/64 is still limited, basic aspects of vector biology should be ex- plored prior to in vivo studies to exclude possible immune evasion mechanisms like inter- ference with antigen processing. Here, the vectors should be compared in a series of ex vivo assays to the well-established vector platforms Ad5 and MVA regarding parameters such as target cell tropism, cytotoxicity as well as antigen expression and presentation (Fig- ure 9). Most parameters should be measured after transduction of dendritic cells (DCs), partly because they are the most potent APCs in vivo. In addition, DCs that are pulsed ex vivo with CMV antigens might be readily applied as a therapeutic vaccine, a strategy which is currently being employed with some success in clinical studies for the treatment of glio- blastomas expressing CMV antigens
190,250.
Figure 9 Transduction of dendritic cells by viral vectors
The CMV antigens IE-1 or pp65 were inserted into the genomes of Modified Vac- cinia Ankara (MVA), Adeno- virus (AdV) and Sendai virus (SeV). After ex vivo trans- duction of human dendritic cells, various parameters like transduction rates, anti- gen expression and presen- tation, cytotoxicity and mat- uration of dendritic cells should be assessed.
27
IV. Results
IV.1. Influence of posttranslational modifications on protein delivery to antigen-presenting cells
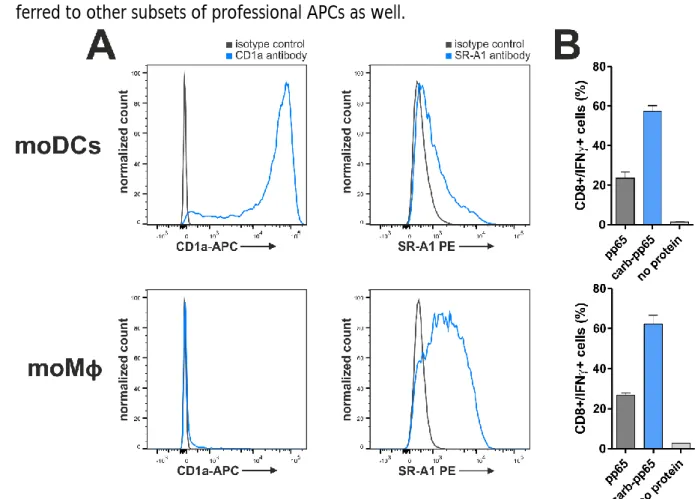
Adaptive immune responses require priming of naïve lymphocytes, a function that is carried out mainly by professional antigen-presenting cells (APCs) like dendritic cells (DCs) and mac- rophages. These cells specialize in taking up proteins from the extracellular space, which are subsequently processed and presented on major histocompatibility (MHC) complexes.
A defining property of professional antigen-presenting cells is the co-expression of MHC class one (MHC-I) and class two (MHC-II) complexes, which allows presentation of peptides to both CD4 and CD8 positive cells, respectively (most cell types express MHC-I only). In addition, professional APCs express a number of costimulatory molecules that activate T cells, thereby engaging multiple independent pathways in parallel and lowering the thresh- old signal required for T cell priming. Due to these properties, professional APCs hold a unique and central role in the initiation of immune responses and interaction with T cells.
As a consequence, techniques that mediate efficient delivery of antigens to these cells have great potential both for the development of novel vaccine candidates (in vivo) as well as monitoring of antigen-specific effector cells (ex vivo).
It was previously reported that storage of the EBV protein BZLF1 in high molar (4 M) urea- solution results in increased stimulation of CD4
+and CD8
+T cells compared to urea-non- treated protein (Figure 10). It was further demonstrated that the urea-treated protein is taken up via clathrin-mediated endocytosis into a number of APCs, including monocyte- derived dendritic cells (moDCs), B cells and monocytes
249.
Figure 10 - Urea treatment in- creases the restimulation of BZLF1-specific T cells
Whole blood from an EBV-seroposi- tive donor was stimulated with BZLF1 or urea-treated BZLF1 (10 µg/ml each) for 7 hours with Bre- feldin A (BFA, 10 µg/ml) present dur- ing the last 4 hours. Cells were stained for CD3, CD4, CD8 and intra- cellular IFN- by flow cy- tometry analysis. Shown is the per- centage of CD3+CD8+ or CD3+CD4+ cells expressing IFN-
sentative stimulation of eight inde- pendent experiments using a total of 4 different donors. Plots show log fluorescence intensity. Modified from Barabas et al. (2008)249.