CHECKLIST AND HOST INDEX OF THE RUST FUNGI (UREDINALES) OF SWITZERLAND
Reinhard Berndt
Thomas Brodtbeck
Checklist and host index of the rust fungi (Uredinales) of Switzerland
June 2020 (vers. 1.0.)
Reinhard Berndt and Thomas Brodtbeck
Adresses of the authors
Reinhard Berndt. ETH Zürich, Institute of Integrative Biology, Universitätstr. 16, 8092 Zürich, Switzerland (reinhard.berndt@env.ethz.ch)
Thomas Brodtbeck. Elsternweg 5, 4125 Riehen, Switzerland (tom.brodtbeck@gmail.com)
Copyright and Citation
This publication is licensed under a Creative Commons license. It may be copied, shared and redistributed in any medium or format under the term that appropriate credit is given to this publication (citation see below). Photographs must not be used for commercial purposes.
Cite as: Berndt R., Brodtbeck T. (2020) Checklist and host index of the rust fungi (Uredinales) of Switzerland. DOI 10.3929/ethz-b-000418925
All photographs by Reinhard Berndt. Front cover: Fruits of Berberis vulgaris with aecia and spermo- gonia of Puccinia graminis. Back cover: Telia of Puccinia asteris on a leaf of Aster sp. Page II (Table of contents): Telia of Melampsora sp. on a poplar leaf (Populus sp.).
Table of contents
ABSTRACTS ... 1
PREFACE ... 2
INTRODUCTION TO RUST FUNGI ... 2
The Swiss rust funga ... 2
Life cycles and spore states ... 4
Determination of rust fungi ... 8
Glossary ... 8
EXPLANATIONS TO THE CHECKLIST ... 10
Names ... 10
Adventive rust species: time and level of establishment ... 11
Hosts ... 11
References and abbreviations used in the text ... 12
LIST OF THE RUST FUNGI RECORDED IN SWITZERLAND ... 13
HOST INDEX ... 89
ACKNOWLEDGEMENTS ... 137
GENERAL REFERENCES ... 137
SPECIFIC REFERENCES ... 141
MORPHOLOGICAL INDEX ... 149
ILLUSTRATIONS ... 151
Abstract
The present checklist of Swiss rust fungi (basidiomycetes, order Uredinales [Pucciniales]) comprises 576 taxa belonging to 543 species. For each taxon, the most important synonyms, the life cycle and the host plants recorded in Switzerland are given. It is indicated, furthermore, whether a taxon is adventive in Switzerland and to what extent it has become naturalized. Information is presented on how the accepted rust species are allotted to the rust genera, the different life cycles and host families. A host-rust index and close-up photographs of 127 species supplement the checklist.
Zusammenfassung
Die vorliegende Checkliste der Schweizer Rostpilze (Basidiomyzeten, Ordnung Uredinales [Pucciniales]) führt 576 Taxa auf, die 543 Arten zugeordnet werden. Für jedes Taxon werden die wichtigsten Synonyme angegeben, der Lebenszyklus und die für die Schweiz nach- gewiesenen Wirtspflanzen. Zusätzlich wird angegeben, ob ein Taxon in der Schweiz adventiv vorkommt und inwieweit es als eingebürgert gelten kann. Eine statistische Analyse zeigt, wie sich die anerkannten Rostpilzarten auf die in der Schweiz vorkommenden Rostpilzgattungen, die verschiedenen Lebenszyklen und die Wirtsfamilien verteilen. Die Checkliste wird durch einen Wirt-Rostpilz-Index und Nahaufnahmen von 127 Arten ergänzt.
Résumé
La présente liste des champignons responsables des rouilles en Suisse (basidiomycètes, ordre des Uredinales [Pucciniales]) comprend 576 taxons appartenant à 543 espèces. Pour chaque taxon sont indiqués les synonymes communs, le cycle de vie et les plantes hôtes suisses. En outre, il est indiqué si un taxon est adventive/exotique en Suisse et dans quelle mesure il s’est acclimaté. Des statistiques portant sur la répartition des espèces répertoriées parmi les différents genres de rouilles, leurs différents cycles de vie et leur association avec les familles de plantes hôtes sont présentées. La liste de contrôle est complétée par un index des combi- naisons plante hôte-rouille et par de gros plans de 127 espèces.
Preface
The latest “Swiss List of National Priority Species and first List of National Priority Habitats”
(BAFU 2019) states that the biological diversity of Switzerland is deteriorating constantly since 1900 and that today the state of the Swiss biodiversity is “unsatisfactory”. The loss of species and the dwindling of populations are well documented for certain conspicuous and well-known organismal groups like vertebrates or vascular plants, while much less is known about the fate of the plethora of inconspicuous or cryptic animals, fungi and other micro- organisms. It is likely that these forgotten organisms are also on the decline, but presently there is little scientific evidence to prove this assumption.
The present checklist is a step on the way to catalogue also the neglected majority of organisms. It deals with microscopically small “microfungi”, specifically the order Uredinales of the basidiomycota, generally known as rust fungi.
BAFU (2019) estimated the number of rust fungi (erroneously equated with the class Pucci- niomycetes) as 420 species in Switzerland. We recognize 576 rust taxa belonging to 543 species – among them a number of exciting new records for the country – and provide infor- mation on their host plants, their life cycle and spore states. In addition, adventive rust fungi are pointed out and their level of naturalization is classified.
The checklist is still incomplete in that it does not give information on the distribution and rarity of the treated species in Switzerland. We consider it as a working version, therefore, and hope that it will allure amateur and professional mycologists to concern themselves with rust fungi and to contribute field records and observations but also to point out omissions and errors. The checklist shall be updated regularly by new information and records and when new scientific results become available.
Introduction to rust fungi The Swiss rust funga
Rust fungi are basidiomycetes that grow as obligate and biotrophic parasites on ferns and seed plants. Some species cause economically important diseases of crops, forest trees or cultivated ornamental plants but the majority of rust fungi occur on wild plants and do not interfere directly with man’s economic interests. Except for their economic importance, rust fungi are well known because of their unusually complicated life cycle involving various spore states and, often, host alternation.
The species diversity and taxonomy of rust fungi have been studied intensively in Switzerland. Fischer (1904) described all Swiss species known at his time in an excellent monograph. Gäumann (1959) authored “Die Rostpilze Mitteleuropas” a monumental and comprehensive work compiling the current taxonomical knowledge about the rust fungi of Switzerland and adjacent regions. Its wide geographic scope makes Gäumann’s book a most useful reference and determination tool for rust fungi of all Central Europe, while it is difficult to extract from it the information that is particular for Switzerland.
The taxonomic and floristic knowledge about Central European rust fungi has grown during the decades that passed since Fischer’s and Gäumann’s books were published, and nomenclature underwent changes as well. Checklists – or even floras – are now available for the adjacent countries Austria (Poelt & Zwetko 1997), Germany (Brandenburger 1994; Braun 1982), and other European countries like Belgium (Vanderweyen & Fraiture 2007, 2008, 2011), Great Britain (Henderson 2000, 2004; Legon & Henrici 2005), the Netherlands (Termorshuizen & Swertz 2011) Poland (Mułenko et al. 2008) and Portugal (Talhinhas et al.
2019). We considered it about time, therefore, to present an up-to-date checklist of the rust fungi occurring on Swiss territory as well.
It is not the task of a checklist to revise the taxonomy of the included species. Nevertheless, when compiling a checklist, one has to ponder on contradictory or competing taxonomic opinions and to decide which one should be followed. One of the most debated questions in rust taxonomy is how species should be delimited and infra-specific entities be defined.
Gäumann (1959) circumscribed rust species narrowly using morphology as the criterion to define “morphospecies” and host specificity to delimit “biological species” also called formae speciales. As both criteria cannot always be separated neatly Gäumann classified his biological species inconsistently as formae speciales or as species (comp. Fischer & Gäumann 1929, p. 151 et seq., p. 206 et seq.). Other uredinologists like Hylander et al. (1953), Wilson
& Henderson (1966) and Cummins (1971) circumscribed rust species more widely and amalgamated closely related biological species in broadly defined morphospecies which can be recognized and named without knowledge about the host specificity.
For a couple of reasons, we decided to include in the present list the narrowly circumscribed species used by Gäumann (1959). One reason is that rusts collected in Switzerland and kept in Swiss herbaria were mainly named according to Gäumann’s monograph. Secondly, central European mycologists continue to use “the Gäumann” as a reference and determination tool.
And thirdly, we believe that it has heuristic value to stick to narrowly defined species in this checklist because this will bring them to the mind of field mycologists who can gather further information on these taxa. On the contrary, by working with species aggregates and by lumping “biological” and “small species” arbitrarily, one risks that “good” taxa will be neglected and information be lost.
We do not consistently follow Gäumann’s species circumscription and nomenclature, however. In a number of cases, where recognizable morphospecies have been atomized into very small entities defined by host preferences we accept more broadly circumscribed species.
We usually treat the small entities that are morphologically virtually indistinguishable as formae speciales. Zwetko (1993) critically remarked that the infection experiments upon which some “biological species” were proposed had not always been carried out and interpreted in a reproducible way. And Anikster (1984) objected that the recognition of formae speciales may depend on the geographic range studied, the regional composition of the flora, and the physiological state of the (potential) host plants.
Based on these considerations we presently recognize 543 species of rust fungi in Switzerland (Table 1) belonging to 30 genera, including two anamorph genera (Table 2).
TABLE 1. General statistics of Swiss rust fungi
Number of recognized rust species 543
Number of infraspecific taxa (excl. autonyms) 33
Number of genera 30 (incl. two anamorph genera)
Ergasio- and ephemerobiontic species 40 (7.4 %)
Species discovered in Switzerland since Gäumann
(1959) 31 (5.7 %)
TABLE 2. Generic partition of Swiss rust fungi
The genera of rust fungi present in Switzerland with the respective species numbers (percentages calculated only for the ten most speciose genera).
Rust genus Number of species
Per cent of total species
Cumulative percentage
Puccinia 335 61.7 61.7
Uromyces 93 17.1 78.8
Melampsora 28 5.2 84.0
Phragmidium 14 2.6 86.6
Milesina 9 1.7 88.3
Gymnosporangium 8 1.5 89.8
Pucciniastrum 7 1.3 91.1
Chrysomyxa 5 0.9 92.0
Tranzschelia 5 0.9 92.9
Thekopsora 4 0.7 93.6
Aecidium (anamorph) 3
Coleosporium 3
Cronartium 3
Hyalopsora 3
Melampsoridium 3
Trachyspora 3
Endophyllum 2
Melampsorella 2
Triphragmium 2
Uredo (anamorph) 2
Cumminsiella 1
Gymnoconia 1
Kuehneola 1
Leucotelium 1
Naohidemyces 1
Nyssopsora 1
Ochropsora 1
Pileolaria (ephemerobiontic) 1
Uredinopsis 1
Xenodochus 1
Life cycles and spore states
Many rust fungi go through complicated life cycles comprising a number of morphologically and functionally different spore states. In this checklist, we use the so-called “ontogenic”
terminology to designate life cycles and spore states. Explanations on this terminology can be found, for example, in Cummins & Hiratsuka (2003). Gäumann (1959), in contrast, adhered to the “morphologic” terminology which differs considerably and is briefly explained in Blumer (1963) or, in some more detail, in Gäumann (1964).
The “complete” or fully expanded life cycle of a rust fungus embraces four spore states called spermogonia, aecia, uredinia and telia. We do not distinguish here basidiospores as a separate spore state because the basidia are formed in the telia. In the present checklist, the life cycle of a rust species is presented as a formula that indicates which of the named spore states are produced, what they look like morphologically, and how they combine. The spore states are designated by the capitals S, A, U, and T as follows:
• S stands for spermogonium/spermogonia,
• A for aecium/aecia,
• U for uredinium/uredinia,
• T for telium/telia.
While some rust species produce all four spore states, others have dropped one or more of them, and still others even have a variable life cycle. The life cycles are designated according to the spore states present:
• Macrocyclic life cycle. The macrocyclic life cycle comprises all spore states. They can be produced on a single host species or on two unrelated host species. Rust fungi of the first group are termed autoecious (formula S A U T), those of the second group heteroecious (formula S A – U T). The regular change between two hosts is called host alternation and is indicated in a formula by inserting an en-dash “–”. The formula S A – U T means that spermogonia and aecia are produced on a different host than the uredinia and telia. The first host is called aecial host, the second one telial host. The macrocyclic, heteroecious life cycle is taught in mycology classes as the standard life cycle of rust fungi using the example of Puccinia graminis, Black Rust of wheat.
• Demicyclic life cycle. Demicyclic rust fungi pruduce spermogonia, aecia and telia on a single or on two host species (formulas S A T or S A – T).
• Microcyclic life cycle. Microcyclic rust fungi form telia accompanied by spermogonia or not. They are always autoecious (formulas S T or T).
• Hemicyclic life cycle. Only uredinia and telia are present (formula U T, rarely UA T).
True hemicyclic rusts are always autoecious. One cannot rule out that there are allegedly hemicyclic rust fungi that produce additional spore states in reality, which have not been discovered so far, however.
• Anamorphic life cycle. Anamorphic rust fungi do not produce spermogonia and telia but only an asexual conidial spore state, usually assignable to the anamorph genus Uredo (formula U) or – rarely – Caeoma or Aecidium (formula A). The spores (coni- dia) are appropriate for spreading the rust fungus to other host plants but not for sexual reproduction.
The life cycles of the Swiss rust fungi are summarized in Table 3. Table 4 presents the arith- metical relationship between the different life cycles and the familial affiliation of the respect- tive host plants.
The capitals used to designate the different spore states can be accompanied by a superscript letter that specifies the morphology of the respective spore state:
• A (without superscript): Aecia showing the characters of the Aecidium-anamorph (in which we include here the Peridermium- and Roestelia-anamorphs).
• AC: Aecia showing the characters of the Caeoma-anamorph (in which we include here the Petersonia-anamorph).
• AU: Aecia showing the characters of the Uredo-anamorph (in which we include here the Milesia-, Physopella-, and Calidion-anamorphs).
• U (without superscript): Uredinia showing the characters of the Uredo-anamorph (incl. Milesia-, Physopella-, and Calidion-anamorphs).
• UA: Uredinia showing the characters of the Aecidium-anamorph (incl. Peridermium- and Roestelia-anamorphs).
• UC: Uredinia showing the characters of the Caeoma-anamorph (incl. Petersonia- anamorph).
• amphi: Indicates the presence of so-called amphispores. A number of rust species form uredinia of the Uredo type in which “ordinary” uredospores are produced toge- ther with or followed by thick-walled and usually more darkly pigmented amphi- spores.
• T (without superscript): “Ordinary” telia. They are composed of pedicellate or sessile teliospores that germinate upon maturity or after a resting period and produce basidia.
Sterile hyphae that bind the sori may occur or not.
• TB: “Basidio-telia”. Teliospores are not formed or are not evident. The telia are com- posed of basidia that produce basidiospores in situ.
TE: “Endo-telia”. Endo-telia look more or less like an Aecidium-anamorph. The spores germinate with basidia, however, and are therefore regarded as teliospores.
Spore states that do not occur regularly in the life cycle of a rust fungus are given in brackets.
In rust fungi with variable life cycle, the known variants are given.
A few examples shall illustrate how the life cycle formulas are read:
S AU U T designates an autoecious macrocyclic rust fungus that produces spermogonia, aecia, uredinia and telia on a single host. The aecia AU have the morphology of the anamorph genus Uredo, as indicated by the superscript.
The formulas S A – T and S AU – T both designate a demicyclic heteroecious rust fungus comprising spermogonia, aecia and telia. The difference is in the morphology of the aecia, which are Aecidium-like in the first case and Uredo-like in the second.
A demicyclic rust fungus with the formula S (A) T produces spermogonia and telia regularly while the aecial state may be lacking or vestigial. Specimens without aecia could be taken for microcyclic.
TABLE 3. Life cycles of Swiss rust fungi
Species numbers of heteroecious and autoecious rust fungi and rust fungi with uncertain life cycles (percentages in brackets). The heter- and autoecious life cycles are further divided into macro-, demi- and microcyclic types. Rust fungi with uncertain life cycle comprise mainly hemicyclic species, anamorphic rusts and and a few variable or incompletely known species.
Host alternation Life cycle species number (percentage) Heteroecious
167 spp. (30.7%)
macrocyclic 157 (28.9%)
demicyclic 10 (1.8%)
Autoecious 288 spp. (52.9%)
macrocyclic 128 (23.3%)
demicyclic 44 (8.1%)
microcyclic 115 (21.1%)
endocyclic 2 (0.4%)
Uncertain 89 spp. (16.4%)
hemicyclic 78 (14.5%)
anamorphic 5 (0.9%)
variable 3 (0.6%)
uncertain 2 (0.4%)
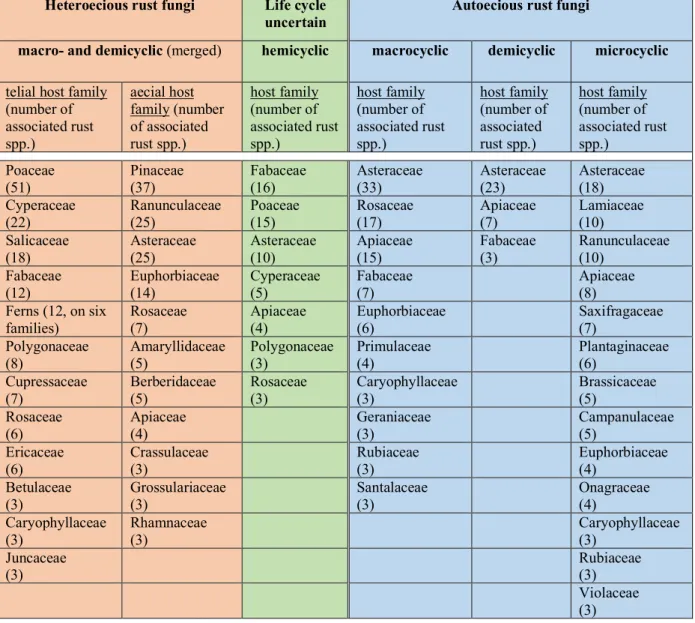
TABLE 4. Host partition of Swiss rust fungi ordered by life cycle
Table 4 shows the numerical relationship between the rust species recorded in Switzerland and their host families. The rust fungi are firstly divided into heter- and autoecious species, and in species with uncertain life cycle. The heter- and autoecious ones are subdivided into the macro-, demi- and microcyclic types. The number of rust species found on a host family is given in brackets. Plant families supporting less than three rust species are not given. For the heteroecious rust fungi, the telial and aecial host families are presented. This distinction was not made in the autoecious and hemicyclic rust fungi that live on a single host species. The hemicyclic rust fungi were provisionally inserted under “uncertain life cycle”. Some of them may be truly hemicyclic, others may just appear hemicyclic but represent other life cycles in reality.
Heteroecious rust fungi Life cycle
uncertain Autoecious rust fungi
macro- and demicyclic (merged) hemicyclic macrocyclic demicyclic microcyclic telial host family
(number of associated rust spp.)
aecial host family (number of associated rust spp.)
host family (number of associated rust spp.)
host family (number of associated rust spp.)
host family (number of associated rust spp.)
host family (number of associated rust spp.)
Poaceae
(51) Pinaceae
(37) Fabaceae
(16) Asteraceae
(33) Asteraceae
(23) Asteraceae
(18) Cyperaceae
(22) Ranunculaceae
(25) Poaceae
(15) Rosaceae
(17) Apiaceae
(7) Lamiaceae
(10) Salicaceae
(18) Asteraceae
(25) Asteraceae
(10) Apiaceae
(15) Fabaceae
(3) Ranunculaceae
(10) Fabaceae
(12)
Euphorbiaceae (14)
Cyperaceae (5)
Fabaceae (7)
Apiaceae (8) Ferns (12, on six
families) Rosaceae
(7) Apiaceae
(4) Euphorbiaceae
(6) Saxifragaceae
(7) Polygonaceae
(8) Amaryllidaceae
(5) Polygonaceae
(3) Primulaceae
(4) Plantaginaceae
(6) Cupressaceae
(7) Berberidaceae
(5) Rosaceae
(3) Caryophyllaceae
(3) Brassicaceae
(5) Rosaceae
(6)
Apiaceae (4)
Geraniaceae (3)
Campanulaceae (5)
Ericaceae
(6) Crassulaceae
(3) Rubiaceae
(3) Euphorbiaceae
(4) Betulaceae
(3) Grossulariaceae
(3) Santalaceae
(3) Onagraceae
(4) Caryophyllaceae
(3)
Rhamnaceae (3)
Caryophyllaceae (3)
Juncaceae
(3) Rubiaceae
(3) Violaceae (3)
Determination of rust fungi
The determination of a rust fungus normally begins with the determination of its host plant.
Because most rust fungi specialize in one or a few host species, knowledge of the host will usually narrow down the possibilities to a couple of rust species or even a single one. To find out which rust fungi are known on the respective host genus or species, the host will need to be looked up in a host index, for example the index provided by this checklist. For a definite and reliable determination, it is necessary, however, to study the rust specimen in question microscopically and to compare the observed characters with the detailed descriptions given in rust floras, monographs and specialized primary literature. Besides the cited works of Fischer (1904) and Gäumann (1959), the rust student may want to consult the excellent Flora of rust fungi of the Mark Brandenburg (Klebahn 1914; in German), the Romanian rust flora (Săvulescu 1953; in Romanian), the Polish rust flora (Majewski 1977, 1979; in Polish), the British rust flora (Wilson & Henderson 1966; in English), and the Dutch rust flora (Termorshuizen & Swertz 2011; in Dutch and English). One must not forget either the four volumes of Monographia Uredinearum (Sydow & Sydow 1904–1924; Latin descriptions, German comments) that are still an indispensable reference for the world’s rust fungi. The named works also provide bibliographies that give access to additional primary and more specialized literature.
There are, in addition, a number of useful illustrated determination guides that cover a broad range of plant parasitic microfungi including rust fungi (e.g. Brandenburger 1985, Ellis &
Ellis 1997, Klenke & Scholler 2015, Viennot-Bourgin 1956).
Illustrations. – Habitus- and close-up photographs are only of limited help in the determi- nation of rust fungi. We provide almost 180 highly resolved photographs of 127 species, nonetheless, to help the reader recognize rust fungi and their different spore states in the field and to become intrigued by the subtle beauty of many of these organisms. The selected species represent most rust genera encountered in Switzerland. They show the different spore states and typical types of rust infection on a broad variety of host families. The Illustrations section starts with a morphological index that helps to find the pictures showing the sought spore state or morphological detail.
Glossary
ad: belonging to
Aecium: Spore state of rust fungi produced upon fertilization of a spermogonium. Aecia produce dikaryotic aeciospores that will either infect the same host species in autoecious rust fungi or alternate to the telial host in heteroecious species. Aecia are represented by different anamorph genera, usually Aecidium, Peridermium, Caeoma or Roestelia.
Aecidium: An anamorph genus. In the Aecidium-anamorph, the spores are dikaryotic, one- celled and produced successively “in chains” by a basal cell. She spore-forming central part of the sorus is surrounded by a sterile bounding layer called peridium. Aecidia are usually cup-shaped and more or less immersed in the host tissue. For simplicity, we subsume under Aecidium the Peridermium- and Roestelia-anamorphs.
Aggregate, collective species: A group of similar and closely related species whose taxonomic rank is often conversely discussed. They may be classified as “small species”
or varieties if distinguishable by morphological differences or as “biological species” (→
forma specialis) when they are separable (almost) exclusively by their host specialization.
Amphispore: Amphispores are modified urediniospores. They have usually a thicker wall and are more darkly pigmented than ordinary urediniospores of the same rust species.
Amphispores likely have the function to persist during adverse seasons, e.g. cold or dry seasons.
Anamorph (adj. anamorphic): A spore forming fungal structure in which spores are produced asexually by mitotic divisions. The resulting spores are termed → conidia. In the rust fungi, the aecial and the uredinial states are anamorphic.
Basionym: The original name to which subsequent names refer by using the same epithet, generally after a change in rank or taxonomic position. For example: Puccinia mucronata Pers. is the basionym of Aregma mucronatum (Pers.) Fr. and of the current name Phrag- midium mucronatum (Pers.) Schltdl.
Caeoma: An anamorph genus. The spores are dikaryotic, one-celled and produced successively “in chains” by a basal cell. She spore-forming part of the sorus is generally not surrounded by a sterile bounding layer or only by an inconspicuous or evanescent one. Caeomata are more or less flat and not immersed into the host tissue. For simplicity, we subsume under Caeoma the Petersonia-anamorph.
Conidium (pl. conidia): Spores that are produced by mitotic cell divisions and can germinate without prior sexual nuclear fusions. In rust fungi, conidia are produced in the aecial and uredinial state. They are formed in morphologically diverse → sori that are named Aecidium, Caeoma or Uredo (among others). Comp. → anamorph.
Funga: The entirety of fungal species occurring in a given region. Analogue of “flora” used for plants and “fauna” for animals.
Forma specialis (pl. formae speciales), or “biological species”: Host specific populations within a rust species that are defined by their host specialization but cannot – or hardly – be distinguished morphologically. For an in-depth discussion of formae speciales see the classical paper by Eriksson (1894), Klebahn (1904) and the review of Anikster (1984).
In scheda: On the label (of a herbarium sheet or package). Refers to a name – often unpub- lished or provisional – written on the label of a herbarium specimen. Not to be confound- ded with names written on so-called revision labels that are attached to herbarium sheets by specialists upon revision of a specimen.
Matrix nova: A new kind of substratum. Here: a new host species of a rust fungus.
Nomen confusum (nom. conf.): A name based on a type consisting of discordant elements from which it is impossible to select a satisfactory lectotype (Jeffrey 1977).
Nomen illegitimum (nom. illegit.), nomen invalidum (nom. inval.): To be applicable, a fungal name has to be validly and legitimately published according to the rules of the International Code of Nomenclature for Algae, Fungi and Plants (“The Code”). Names violating the rules of the Code are either invalid (nomina invalida) or illegitimate (nomina illegitima).
Nomen nudum (nom. nud.): A name published without the descriptive matter necessary to satisfy the requirements of the appropriate nomenclatural Code for valid publication (Jeffrey 1977).
Nomen provisorium (nom. provis.): Provisional, unpublished name.
Paraphysis (pl. paraphyses): Sterile dikaryotic hyphal elements that are produced in the sori (generally uredinia or telia) of certain rust fungi. Paraphyses are most often clavate, cylindrical or capitate. They may be restricted to the border of the sori or can be scattered among the spores.
Sensu: in the sense of; as used by. For example, the phrase “Puccinia valida Bauer sensu Gäumann” means that Gäumann uses the name P. valida differently than the original author of the species, Bauer.
Small species: Small species are species that differ by minor but constant morphological traits from similar species. Groups of related small species are sometimes termed → aggregates or collective species. Alternatively, related small species may not be regarded as species but as varieties of a single species. This decision is arbitrary.
Sorus (pl. sori): A spore producing hyphal weave with limited growth and well-defined morphology. The term is mainly used for rust and smut fungi. The terminology of the sori of rust fungi is explained in section “Life cycle and spore states”.
Spermogonium, also called pycnidium: Spermogonia are formed by the haploid mycelium that results from infection of a host plant by basidiospores. Within a spermogonium numerous spermatia are produced that serve as gametes and initiate the formation of the dikaryotic mycelium.
Synonym: Any alternative name applied to a → taxon.
Taxon (pl. taxa): Any taxonomic entity named in the biological system of organisms – irrespective of the taxonomic rank. For example, the Pucciniaceae is a taxon with the rank of family. It includes the taxon Puccinia graminis with species rank. Puccinia graminis comprises a number of infraspecific taxa, like P. graminis ssp. graminicola, that has the rank of a subspecies.
Telium: Spore state of rust fungi producing teliospores. Teliospores, also called probasidia, are initially dikaryotic (possessing two compatible haploid nuclei). The haploid nuclei of a teliospore cell merge to form a diploid nucleus which undergoes meiosis when basidia are formed. Teliospores are morphologically diverse. They can be one- to multi-celled and are borne by pedicels or sessile.
Uredinium: Spore state of rust fungi following upon the aecial state. Uredinia are most often represented by the anamorph genus Uredo s. l. They produce dikaryotic spores that are able to re-infect the telial host (“anamorphic cycle”). Uredinia are followed by the telial spore state.
Uredinologist: A mycologist specializing in the basidiomycete order Uredinales, the rust fungi.
Uredo: An anamorph genus. In the Uredo-anamorph, the spores are dikaryotic, one-celled and produced singly on pedicels. For simplicity, we subsume under Uredo the Milesia-, Physopella-, and Calidion-anamorphs which are basically similar but differ in the pre- sence of binding hyphae.
Explanations to the checklist
The present checklist is primarily based on Fischer (1904), Gäumann (1959) and more recent- ly published primary literature listed in the “Specific references” section. Specific references are given in the list only for the rust species discovered in Switzerland after the publication of Gäumann (1959). In addition to publications, we mined the data of the rust fungi kept in the United Herbaria of University and ETH Zürich (Rostpilze der Zürcher Herbarien [without date]), while the rust collections of the herbaria of Geneva and Neuchâtel have not been evaluated so far. Finally, we supplemented the list with numerous new records from our own field observations throughout Switzerland, especially the ones made by Thomas Brodtbeck.
Each entry in the checklist comprises (1) the accepted name, (2) important synonyms, (3) information on the status as adventive species (if applicable), (4) the known Swiss host plants, and (5) specific notes (where appropriate). Rust taxa not included in Gäumann (1959) and those illustrated by a photograph are pointed out.
Names
The rust fungal genera and species are listed alphabetically.
• Fungal names that we accept as correct and actual are printed in bold-italic. Where we consider it helpful, we provide commonly used or other important synonyms of the
accepted names. For an exhaustive list of synonyms, the reader should refer to online ressources like Index Fungorum (http://www.indexfungorum.org/Names/Names.asp) or MycoBank (http://mycobank.org) or to the manuals of Sydow & Sydow (1904–
1924), Wilson & Henderson (1966), Legon & Henrici (2005), and Termorshuizen &
Swertz (2011). Boerema & coworkers (1993) is also most useful for finding correct names of common rust fungi of central Europe.
• Names regarded as actual and valid by Gäumann (1959) but considered as synonyms by us are given in normal italics and marked with an ★asterisk. We include these names both in the main list and in the synonymy to facilitate cross-referencing with Gäumann’s manual.
• Names of species aggregates – or collective species – are underlined and in italics.
The related “small species” accepted by us are listed in bold-italic.
• Rust fungi that have been reported for Switzerland erroneously or most likely erron- eously are given in [square brackets].
Adventive rust species: time and level of establishment
Following the life cycle formulas, information is given on adventive rust fungi.
The native Swiss rust funga has become enriched by adventive species since the neolithic era (ca. 7500 before present in Switzerland). We regard a rust species as adventive when it was likely introduced together with an adventive host species. If possible, we try to distinguish between archaeo-adventive species that reached central Europe since the neolithic era and neo-adventive species which did not arrive before 1500 a.d. We use the term adventive in a wide sense for both rust species that were likely spread together with their hosts by human action (i.e. anthropochorously) and for those who followed their adventive hosts without demonstrable human influence (i.e. idiochorously).
It is important to note that adventive rust fungi need not remain restricted to their original hosts after introduction into their new range but may be able to spread to native hosts, usually taxonomically related to the adventive ones. On the other hand, adventive plants may be colo- nized by resident rust species by host range expansions of the latter.
To classify the level of establishment of an adventive rust species, we use the following cate- gories:
Agriobiont (agr): Firmly established on introduced (or native) hosts in the wild.
Ephemerobiont (eph): Not firmly established; appearing and vanishing depending on the ephemeral occurrence of the respective host plants. We subsume under ephemerobiontic the accidental appearance of alien rust fungi in gardens or green houses, etc.
Ergasiobiont (erg): Restricted to cultivated crops and ornamentals.
For example: Puccinia malvacearum originates from South America and came to Europe after 1500 a.d. It has become firmly established since on a broad variety of cultivated and wild Malvaceae. We categorize it, therefore, as a neo-adventive agriobiont (neo-adv agr).
Data on neo-adventive rust fungi were partly taken from Beenken & Senn-Irlet (2016).
Hosts
For each accepted rust species (or infraspecific taxon) we name the genera and species of host plants on which the respective fungus has been recorded in the wild. Plant species whose susceptibility to a given rust species was only ascertained by infection experiments are gene-
rally not listed. The names of the host plants accord to the Checklist of Vascular Plants of Switzerland (Juillerat et al. 2017).
In heteroecious rust fungi, the telial hosts are preceded by a T, the aecial hosts by an A.
Condensed references and abbreviations used in the text
(Full citations of the references are given in the References section)
B & Co 1993: Boerema & coworkers (1993). Check-lists for scientific names Cu 1971: Cummins (1971). Rust Fungi on Grasses
Gm 1959: Gäumann (1959). Rostpilze der Schweiz
Ind Fung: Index Fungorum website (http://www.indexfungorum.org/Names/Names.asp) M & U 1998: Marková & Urban (1998). Rust Fungi of Grasses in Europe VI
P & Z 1997: Poelt & Zwetko (1997). Rostpilze Österreichs
Syd: Sydow & Sydow (1904–1924). Monogaphia Uredinearum Vol. I–IV T & S 2011: Termorshuizen & Swertz (2011). Dutch Rust Fungi
W & H 1966: Wilson & Henderson (1966). British Rust Fungi
★: Names preceded by an asterisk were regarded as actual and valid by Gäumann (1959). We use an alternative name for them.
▲: Rust species marked by “▲” were recorded in Switzerland for the first time after the publication of Gäumann (1959)
agg.: Aggregate, or collective species
adv: Adventive rust species (see section “Adventive rust species”) agr: Agriobiont (see section “Adventive rust species”)
archaeo-adv: archaeo-adventive rust species CH: Switzerland
Comp.: Compare
cult. (cultivated): Indicates that a host species is alien to the Swiss flora and occurs only in cultivation
cv.: Cultivar. A variety derived from breeding
eph: Ephemerobiont (see section “Adventive rust species”) erg: Ergasiobiont (see section “Adventive rust species”) et seq.: et sequens, and the following
in hort. / in hort. bot. (in horto, hortis / in horto botanico, hortis botanicis): In garden(s); in botanical garden(s)
neo-adv: Neo-adventive rust species (see section “Adventive rust species”)
Phot: Indicates that a photograph of the rust fungus is provided in the Illustrations section p.p.: pro parte, in part
RB: Reinhard Berndt sp.: Species (sgl.) spp.: Species (pl.) Syn.: Synonym
TB: Thomas Brodtbeck
Z+ZT: United herbaria of the University Zürch (Z) and ETH Zürich (ZT).
List of the rust fungi recorded in Switzerland
Aecidium (anamorph state) Aecidium molluginis Wurth Life cycle/spore states: A
Host(s): Galium: G. lucidum, G. mollugo (Rubiaceae).
NOTES: Aecidium molluginis likely represents the aecial state of a heteroecious rust fungus whose identity is unknown (Gm 1959).
Aecidium scabiosae (Dozy & Molk.) G. Winter (Syn.: A. succisae L.A. Kirchn.) Life cycle/spore states: S A
Host(s): Knautia: K. arvensis, K. sylvatica (Caprifoliaceae – “Dipsacaceae”).
NOTES: Aecidium scabiosae may represent the aecial state of a heteroecious rust fungus whose identity is unknown (Gm 1959).
Aecidium tranzschelianum Lindr. ▲, Phot Life cycle/spore states: S A
Host(s): Geranium: G. sanguineum (Geraniaceae).
NOTES: Aecidium tranzschelianum was discovered in canton Valais in 2017 (RB). It has been recorded before only from very few localities in Russia, Germany and Austria. The life cycle of A. tranzschelianum is unknown. We found it in the vicinity of Puccinia pedunculata in which only uredinia and telia are known. Our hypothesis that both taxa might represent the same species was not supported by a nDNA sequence analysis which revealed that both rusts had a dissimilar ITS region (M. García Otálora, unpubl. results).
Calyptospora à Pucciniastrum
★Calyptospora goeppertiana J.G. Kühn (Syn.: Pucciniastrum goeppertianum (J.G. Kühn) Kleb.).
Chrysomyxa
Chrysomyxa abietis (Wallr.) Unger Life cycle/spore states: T
Host(s): Picea: P. abies (Pinaceae).
Chrysomyxa empetri (Pers.) J. Schröt.
Life cycle/spore states: S A – UC T
Host(s): T Empetrum: E. nigrum (Ericaceae). A Picea: P. abies (Pinaceae).
Chrysomyxa pyrolata (Körn.) G. Winter Life cycle/spore states: S A – UC T
Host(s): T Pyrola: P. chlorantha, P. media, P. minor, P. rotundifolia (Ericaceae). A Picea: P.
abies (Pinaceae).
Chrysomyxa ramischiae Lagerh.
Life cycle/spore states: S A – UC T
Host(s): T Orthilia: O. secunda (Ericaceae). A Picea: P. abies (Pinaceae).
Chrysomyxa rhododendri (DC.) de Bary, Phot Life cycle/spore states: S A – UC T
Host(s): T Rhododendron: R. ferrugineum, R. hirsutum, also on their hybrid R. × intermedium (Ericaceae). A Picea: P. abies (Pinaceae).
Coleosporium
★Coleosporium cacaliae (DC.) G.H. Otth (Syn.: C. tussilaginis (Pers.) Berk. f. sp. senecionis- silvatici p.p.; ★C. senecionis Fr. & Kickx).
Life cycle/spore states: S A – UC TB
Host(s): T Adenostyles: A. alliariae, A. glabra (Asteraceae). A Pinus: P. mugo s. l., P. nigra, P. sylvestris (Pinaceae).
★Coleosporium campanulae (Str.) Tul. (Syn.: C. tussilaginis (Pers.) Berk. f. sp. campanulae- rapunculoidis).
Coleosporium cf. montanum (Arthur & F. Kern) McTaggart & Aime ▲, Phot Life cycle/spore states: S A – UC TB (neo-adv erg)
Host(s): T Aster (Symphyotrichum): A. novae-angliae (Asteraceae). A Pinus (Pinaceae), host alternation apparently unknown in CH.
NOTES: Aster novae-angliae bearing uredinia and telia of a Coleosporium sp. was collected in CH in 2019, in a garden center in Dürnten (RB). The rust was determined as C. montanum based on the morphological characters of the telia and on the host identity. The teliospores of the Swiss specimen measured 65–105 × 19–23 µm and were partly borne by distinct “basal cells”. Coleosporium delicatulum (Arthur & F. Kern) Hedgc. & Long occurs on A. novae- angliae as well but has shorter teliospores that lack basal cells (McTaggart & Aime 2018, Weir 1925). The presence of basal cells supporting the teliospores is an ambiguous character.
The subhymenial plectenchyma of the telia has an intricate texture in which the presence or absence of basal cells can easily be misinterpreted.
Ludwig Beenken (in litt.) informed us that he recently collected C. montanum in the botanical garden of Konstanz (Germany) on Solidago canadensis and S. gigantea. The authors do not know of any other reports of C. montanum from outside of North America. It cannot be ruled out, however, that the fungus has been recorded already under the name C. solidaginis which has been used until recently in a wide sense embracing C. montanum. It should be noted that Hedgcock used the name “C. montanum (Arthur & F. Kern) Hedgc.” already in 1933 (Plant Disease Reporter 17(3), p. 24) though this combination may not have been proposed in accordance with the regulations of the Code of Botanical Nomenclature.
Because C. montanum is not covered by European rust floras and checklists, we provide a close-up photograph and micrographs of the telio- and urediniospores.
★Coleosporium pulsatillae (Steud.) Lév. (Syn.: C. tussilaginis (Pers.) Berk. f. sp. pulsatillae).
★Coleosporium senecionis Fr. & Kickx (Syn.: C. tussilaginis (Pers.) Berk. f. sp. senecionis- silvatici p.p.; ★C. cacaliae (DC.) G.H. Otth).
Life cycle/spore states: S A – UC TB
Host(s): T Senecio: S. alpinus, S. doronicum, S. fuchsii, S. inaequidens, S. nemorensis, S.
sylvaticus, S. viscosus, S. vulgaris (Asteraceae). A Pinus: P. mugo s. l., P. sylvestris (Pinaceae).
Coleosporium solidaginis (Schwein.) Thüm. ▲ (Basionym: Uredo solidaginis Schwein.
Alleged synonym: C. asterum (Dietel) Syd. & P. Syd.), Phot
Life cycle/spore states: S A – UC TB (neo-adv erg)
Host(s): T Callistephus: C. chinensis?; Solidago: S. gigantea, S. virgaurea (Asteraceae). A Pinus (Pinaceae), host alternation apparently unknown in CH.
NOTES: It has been known for long that C. solidaginis occurs in North America in two forms that differ in morphology, aecial host and geographic range. These forms have usually been referred to as “eastern” and “western form” of goldenrod rust (e.g. Weir 1925, Ziller 1974).
The western form is nowadays known as C. montanum, the eastern one as C. solidaginis s. str.
(McTaggart & Aime 2018). Coleosporium solidaginis was discovered in canton Ticino in 2012 by TB (Brodtbeck 2017) and has since been found in various other cantons on both S.
gigantea and virgaurea. Beenken & Senn-Irlet (2016) claimed that C. solidaginis had been transmitted from the neophytic Solidago gigantea to the native S. virgaurea.
It is uncertain whether the rust on Callistephus chinensis is really C. solidaginis s. str. It might rather represent C. montanum from western North America or C. asterum, a native of eastern Asia (comp. Hiratsuka et al. 1992).
★Coleosporium sonchi (Schumach.) Lév. (Syn.: C. tussilaginis (Pers.) Berk. f. sp. sonchi).
★Coleosporium tussilaginis (Pers.) Berk. s. str. (Syn.: C. tussilaginis f. sp. tussilaginis).
Coleosporium tussilaginis (Pers.) Berk. f. sp. campanulae-rapunculoidis (Syn.: ★C.
campanulae (Str.) Tul.), Phot Life cycle/spore states: S A – UC TB
Host(s): T Campanula: C. barbata, C. bononiensis, C. cervicaria, C. cochleariifolia, C. glo- merata, C. latifolia, C. medium, C. patula, C. persicifolia, C. rapunculoides, C. rapunculus, C. rhomboidalis, C. rotundifolia, C. scheuchzeri, C. thyrsoides, C. trachelium; Legousia: L.
speculum-veneris; Phyteuma: P. betonicifolium, P. orbiculare, P. ovatum, P. scheuchzeri, P.
spicatum (Campanulaceae). A Pinus: P. mugo s. l., P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Klebahn (1904). Comp. Boerema & Verhoeven (1972) and Helfer (2013). Phyteuma orbiculare and ovatum are new hosts for CH (TB).
Coleosporium tussilaginis (Pers.) Berk. f. sp. inulae (Syn.: ★C. inulae Rabenh.), Phot Life cycle/spore states: S A – UC TB
Host(s): T Inula: I. helvetica, I. hirta, I. salicifolia, I. spiraeifolia (in hort. bot. Bern, TB) (Asteraceae). A Pinus: P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Helfer (2013).
Coleosporium tussilaginis (Pers.) Berk. f. sp. melampyri (Syn.: ★C. melampyri (Rebent.) P.
Karst.), Phot
Life cycle/spore states: S A – UC TB
Host(s): T Melampyrum: M. arvense, M. cristatum, M. nemorosum, M. pratense, M. sylvati- cum (Orobanchaceae). A Pinus: P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Boerema & Verhoeven (1972). Comp. Helfer (2013).
Coleosporium tussilaginis (Pers.) Berk. f. sp. petasitis (Syn.: ★C. petasitis Cooke).
Life cycle/spore states: S A – UC TB
Host(s): T Petasites: P. albus, P. hybridus, P. paradoxus (Asteraceae). A Pinus: P. mugo s. l., P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Boerema & Verhoeven (1972). Comp. Helfer (2013).
Coleosporium tussilaginis (Pers.) Berk. f. sp. pulsatillae (Syn.: ★C. pulsatillae (Steud.) Lév.).
Life cycle/spore states: S A – UC TB
Host(s): T Pulsatilla: P. vulgaris (Ranunculaceae). A Pinus (Pinaceae), host alternation apparently unknown in CH.
NOTES: This forma specialis was proposed by Boerema & Verhoeven (1972). Comp. Helfer (2013).
Coleosporium tussilaginis (Pers.) Berk. f. sp. rhinanthacearum (Syn.: ★C. euphrasiae (Schumach.) G. Winter).
Life cycle/spore states: S A – UC TB
Host(s): T Euphrasia: E. hirtella, E. nemorosa, E. picta, E. rostkoviana, E. salisburgensis, E.
stricta; Odontites: O. luteus, O. vernus, O. vulgaris; Rhinanthus: R. alectorolophus, R. gla- cialis, R. minor, R. serotinus (= angustifolius) (Orobanchaceae). A Pinus: P. mugo s. l., P.
nigra, P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Boerema & Verhoeven (1972). Comp. Helfer (2013). Rhinanthus angustifolius (sub R. serotinus) may be a new host plant for CH (TB).
Coleosporium tussilaginis (Pers.) Berk. f. sp. senecionis-silvatici (Syn.: ★C. cacaliae (DC.) G.H. Otth; ★C. senecionis Fr. & Kickx), Phot
Life cycle/spore states: S A – UC TB
Host(s): T Adenostyles: A. alliariae, A. glabra; Senecio: S. alpinus, S. doronicum, S. fuchsii, S. inaequidens, S. nemorensis, S. sylvaticus, S. viscosus, S. vulgaris (Asteraceae). A Pinus: P.
mugo s. l., P. nigra, P. sylvestris (Pinaceae).
NOTES: This forma specialis was proposed by Wagner ex Gäumann (1959). Comp. Boerema
& Verhoeven (1972) and Helfer (2013). Observed since 2007 on S. inaequidens in CH (TB).
Coleosporium tussilaginis (Pers.) Berk. f. sp. sonchi (Syn.: ★C. sonchi (Schumach.) Lév.).
Life cycle/spore states: S A – UC TB
Host(s): T Sonchus: S. arvensis, S. asper, S. oleraceus, S. palustris (Asteraceae). A Pinus (Pinaceae), host alternation apparently unknown in CH.
NOTES: This forma specialis was proposed by Boerema & Verhoeven (1972). Comp. Helfer (2013).
Coleosporium tussilaginis (Pers.) Berk. f. sp. tussilaginis (Syn.: ★C. tussilaginis (Pers.) Berk.
s. str.).
Life cycle/spore states: S A – UC TB
Host(s): T Tussilago: T. farfara (Asteraceae). A Pinus: P. sylvestris (Pinaceae).
Cronartium
Cronartium flaccidum (Alb. & Schwein.) G. Winter, Phot Life cycle/spore states: S A – U T
Host(s): T Vincetoxicum: V. hirundinaria, V. nigrum (in hort. bot. Brüglingen, TB) (Apocy- naceae); Melampyrum: M. sylvaticum (Orobanchaceae); Paeonia: P. officinalis (in natura?), Paeonia spp. (in hort.) (Paeoniaceae). A Pinus: P. sylvestris (Pinaceae).
NOTES: Melampyrum sylvaticum, on which the rust was found in canton Sankt Gallen in 2019 (RB), seems to be a new host species of C. flaccidum.
Cronartium quercuum (Berk.) Miyabe Life cycle/spore states: S A – U T
Host(s): T Quercus: Q. robur (Fagaceae). A Pinus (Pinaceae), host alternation unknown in CH.
Cronartium ribicola J.C. Fischer, Phot
Life cycle/spore states: S A – U T (neo-adv agr)
Host(s): T Ribes: R. alpinum, R. aureum, R. nigrum, R. petraeum, R. rubrum, R. sanguineum, R. uva-crispa (Grossulariaceae). A Pinus: P. cembra, P. strobus (Pinaceae).
Cumminsiella
Cumminsiella mirabilissima (Peck) Nannf., Phot Life cycle/spore states: S A U T (neo-adv erg/agr) Host(s): Mahonia: M. aquifolium (Berberidaceae).
Endophyllum
Endophyllum euphorbiae-sylvaticae (DC.) G. Winter Life cycle/spore states: S TE
Host(s): Euphorbia: E. amygdaloides (Euphorbiaceae).
Endophyllum sempervivi (Alb. & Schwein.) de Bary, Phot Life cycle/spore states: S TE
Host(s): Sempervivum: S. alpinum, S. arachnoideum, S. grandiflorum, S. montanum, S.
tectorum, S. wulfenii (Crassulaceae).
Frommea à Phragmidium
★Frommea obtusa (F. Strauss) Arthur (Syn.: Phragmidium tormentillae Fuckel; Frommeëlla tormentillae (Fuckel) Cummins & Hirats.).
★Frommea obtusa-duchesneae Arthur (Syn.: Frommeëlla mexicana (Mains) J.W. McCain &
J.F. Hennen var. indica McCain & J.F. Hennen; Phragmidium duchesneae P. Syd. & Syd.).
Gymnoconia
Gymnoconia nitens (Schwein.) F. Kern & Thurst.
Life cycle/spore states: S AC T
Host(s): Rubus: R. saxatilis (Rosaceae).
Gymnosporangium
Gymnosporangium amelanchieris E. Fisch. ex F. Kern (Syn.: G. juniperinum auct., fide Gm 1959).
Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Amelanchier: A. ovalis (Rosaceae).
Gymnosporangium clavariiforme (Pers.) DC., Phot Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Amelanchier: A. ovalis; Crataegus: C.
monogyna, C. laevigata; Sorbus: S. aucuparia (Rosaceae).
Gymnosporangium confusum Plowr.
Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Cotoneaster: C. integerrimus, C.
tomentosus; Sorbus: S. aucuparia (Rosaceae).
Gymnosporangium cornutum (Pers.) Arthur (Syn.: G. juniperinum (L.) Fr.; ★G. torminali- juniperinum E. Fisch. ex F. Kern?), Phot
Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Amelanchier: A. ovalis; Sorbus: S.
aucuparia, S. × hybrida (Rosaceae).
NOTES: May not differ specifically from G. torminali-juniperinum which is regarded as a forma specialis of G. juniperinum auct. (= G. cornutum) by Gm 1959.
Gymnosporangium fusisporum E. Fisch.
Life cycle/spore states: S A – T
Host(s): T Juniperus: J. sabina (Cupressaceae). A Cotoneaster: C. integerrimus, C. tomen- tosus; Sorbus: S. aucuparia (Rosaceae).
NOTES: Gm 1959 regards G. fusisporum as a forma specialis of G. confusum.
Gymnosporangium gaeumannii Zogg Life cycle/spore states: U T
Host(s): Juniperus: J. communis (Cupressaceae).
Gymnosporangium sabinae (Dicks.) G. Winter, Phot Life cycle/spore states: S A – T
Host(s): T Juniperus: J. chinensis (cult.), J. sabina, J. virginiana (cult.) (Cupressaceae). A Pyrus: P. communis, P. pyraster, P. nivalis (in hort. bot. Bern, TB) (Rosaceae).
★Gymnosporangium torminali-juniperinum E. Fisch. ex F. Kern (Syn.: G. cornutum (Pers.) Arthur?).
Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Sorbus: S. torminalis (Rosaceae).
Gymnosporangium tremelloides (A. Br.) R. Hartig Life cycle/spore states: S A – T
Host(s): T Juniperus: J. communis (Cupressaceae). A Malus: M. domestica, M. floribunda (cult.), M. sylvestris; Sorbus: S. aria, S. aucuparia, S. chamaemespilus, S. hostii, S. latifolia, S. mougeotii, S. torminalis, S. diverse hybrids (Rosaceae).
NOTES: Malus floribunda and S. mougeotii are apparently new host species for CH (TB).
Hyalopsora
Hyalopsora adianti-capilli-veneris (DC.) Syd. & P. Syd. ▲ Life cycle/spore states: U T
Host(s): T Adiantum: A. capillus-veneris (Pteridaceae). A Uncertain, likely Abies (Pinaceae).
NOTES: Recently discovered in canton Ticino by Ludwig Beenken (Winterthur). Amphispo- res are apparently unknown (Hiratsuka 1958).
Hyalopsora aspidiotus (Peck) Magnus, Phot Life cycle/spore states: S A – U(+amphi) T
Host(s): T Gymnocarpium: G. dryopteris, G. robertianum (Woodsiaceae). A Abies: A. alba (Pinaceae).
Hyalopsora polypodii (Pers.: Dietel) Magnus, Phot Life cycle/spore states: U(+amphi) T
Host(s): T Cystopteris: C. alpina, C. dickieana (matrix nova), C. fragilis (Woodsiaceae). A Uncertain, likely Abies (Pinaceae).
NOTES: We are unaware of any prior collections of H. polypodii on the rare C. dickieana which is therefore regarded as a new host species of this rust fungus (discovered in canton Valais in 2017, RB).
Kuehneola
Kuehneola uredinis (Link) Arthur, Phot Life cycle/spore states: S AU U T
Host(s): Rubus: R. fruticosus s. l., R. laciniatus (Rosaceae).
Leucotelium
Leucotelium cerasi (Béreng.) Tranzschel, Phot Life cycle/spore states: S A – U T (neo-adv agr)
Host(s): T Prunus: P. avium, P. cerasifera cv. Pissardii, P. cerasus, P. domestica, P. insititia, P. padus, P. spinosa (Rosaceae). A Eranthis: E. hiemalis (Ranunculaceae).
NOTES: In CH, telia of L. cerasi are usually found rather late in the year (September, October) when the uredinia have already vanished. The telia are frequently encountered, however, together with uredinia – and telia – of Tranzschelia discolor or T. pruni-spinosae on the same host leaves. Uredinia of Tranzschelia can be distinguished microscopically from Leucotelium by the the presence of paraphyses and a couple of spore characters.
Melampsora
Melampsora abieti-caprearum Tubeuf (A member of the M. epitea agg.), Phot S AC – U T
Host(s): T Salix: S. appendiculata, S. caprea, S. cinerea (Salicaceae). A Abies: A. alba (Pinaceae).
Melampsora allii-fragilis Kleb. (incl. ★M. galanthi-fragilis Kleb. Comp. Bagyanarayana 2005).
Life cycle/spore states: S AC – U T
Host(s): T Salix: S. fragilis, S. pentandra (Salicaceae). A Allium: A. oleraceum, A. ursinum, A. vineale (Amaryllidaceae).
Melampsora allii-populina Kleb.
Life cycle/spore states: S AC – U T
Host(s): T Populus: P. canadensis, P. nigra (Salicaceae). A Allium: A. carinatum, A. ursinum (Amaryllidaceae); Arum: A. maculatum (Araceae).
★Melampsora allii-salicis-albae Kleb. (Syn.: M. salicis-albae Kleb.).
Melampsora alpina Juel (A member of the M. epitea agg. Syn.: M. arctica Rostr., fide Bagyanarayana (2005); M. epitea Thüm. f. sp. arctica (Rostr.) Bagyan.), Phot
Life cycle / spore states: S AC – U T
Host(s): T Salix: S. breviserrata, S. herbacea, S. retusa (Salicaceae). A Saxifraga: S.
moschata, S. oppositifolia (Saxifragaceae).
Melampsora amygdalinae Kleb.
Life cycle/spore states: S AC U T Host(s): Salix: S. triandra (Salicaceae).
Melampsora capraearum Thüm. (Syn.: ★M. larici-caprearum Kleb.).
Life cycle/spore states: S AC – U T
Host(s): T Salix: S. appendiculata, S. aurita, S. caprea, S. cinerea, S. × smithiana (Salica- ceae). A Larix: L. decidua (Pinaceae).
NOTES: The different spellings “caprearum” and “capraearum” are bothersome. The name refers to Salix section Capreae that includes S. caprea. We use the spellings as originally pub- lished.
Melampsora epitea Thüm. (Likely a species aggregate. The “small species” – or formae speciales – are treated separately to conform to Gäumann 1959: M. abieti-caprearum, M.
alpina, M. euonymi-caprearum, M. laricis-epitea, M. repentis).
Life cycle/spore states: S AC – U T
Host(s): T Salix (Salicaceae). A Various genera belonging to different plant families, depending on species circumscription.
NOTES: Zhao et al. (2017) accept M. epitea s. str. and chose Thümen’s original illustrations as lectotype on the type host, Salix viminalis. We object, however, that neither Thümen’s description nor his illustrations allow to distinguish M. epitea from morphologically similar Melampsoras on Salix. We treat the “small species” listed above separately, therefore, and use the name M. epitea only to designate the whole species aggregate.
Melampsora euonymi-caprearum Kleb. (A member of the M. epitea agg. Syn.: M. epitea Thüm. f. sp. euonymi-capraearum (Kleb.) Boerema & Verhoeven).
Life cycle/spore states: S AC – U T
Host(s): T Salix: S. cinerea, S. eleagnos (Salicaceae). A Euonymus: E. europaeus, E. latifolius (Celastraceae).
Melampsora euphorbiae (Schub.) Cast., Phot Life cycle/spore states: S AC U T
Host(s): Euphorbia: E. cyparissias, E. esula, E. exigua, E. palustris, E. peplus, E.
polychroma, E. verrucosa (Euphorbiaceae).
NOTES: Gm 1959 distinguished three “biological species” – or formae speciales – that we do not treat separately. We regard M. euphorbiae-amygdaloides as a forma specialis of M.
euphorbiae.
Melampsora euphorbiae-amygdaloidis W. Müll. (ad M. euphorbiae) Life cycle/spore states: U T
Host(s): Euphorbia: E. amygdaloides (Euphorbiaceae).
NOTES: Müller (1907) named this rust non-uniformly as “forma specialis” or as species (M.
euphorbiae-amygdaloides). The name M. euphorbiae-amygdaloides is based on the uredinial state of which Müller did not give a formal description. It is hence invalid. We suggest assign- ing M. euphorbiae-amygdaloidis to M. euphorbiae as a forma specialis.
Melampsora euphorbiae-dulcis G.H. Otth
Life cycle/spore states: S AC U T
Host(s): Euphorbia: E. carniolica, E. dulcis, E. lathyris, E. platyphyllos, E. stricta (Euphorbiaceae).
Melampsora euphorbiae-gerardianae W. Müll.
Life cycle/spore states: S AC U T
Host(s): Euphorbia: E. seguieriana (= gerardiana) (Euphorbiaceae).
NOTES: Müller (1907) suggested a possible affiliation of his provisional “Melampsora eu- phorbiae amydaloides” with M. gelmii- or M. euphorbiae-gerardianae-type melampsoras. We suggest assigning it to M. euphorbiae. Melampsora gelmii is not accepted as a member of the Swiss rust funga.
★Melampsora galanthi-fragilis Kleb. (Syn.: M. allii-fragilis Kleb.).
Melampsora helioscopiae (Pers.) Cast., Phot Life cycle/spore states: S AC U T
Host(s): Euphorbia: E. helioscopia (Euphorbiaceae).
Melampsora hypericorum G. Winter (Syn.: Mesopsora hypericorum (DC.) Dietel), Phot Life cycle/spore states: UC T
Host(s): Hypericum: H. androsaemum (cult., TB), H. calycinum? (cult.), H. coris, H. hirsu- tum, H. humifusum, H. maculatum, H. montanum, H. pulchrum, H. perforatum, H. richeri, H.
spp. (cult.) (Hypericaceae).
NOTES: It is uncertain whether the common Caeoma state encountered on cultivated H.
calycinum belongs to the present species.
★Melampsora laricis-caprearum Kleb. (Syn.: M. capraearum Thüm.).
Life cycle/spore states: S AC – U T
Host(s): T Salix: S. appendiculata, S. aurita, S. caprea, S. cinerea, S. × smithiana (Salica- ceae). A Larix: L. decidua (Pinaceae).
Melampsora laricis-epitea Kleb. (A member of the M. epitea agg.), Phot Life cycle/spore states: S AC – U T
Host(s): T Salix: S. appendiculata, S. aurita, S. babylonica (cult.), S. bicolor, S. breviserrata, S. caesia, S. caprea, S. cinerea, S. daphnoides, S. eleagnos, S. foetida, S. glabra, S. glauca, S.
glaucosericea, S. hastata, S. hegetschweileri, S. helvetica, S. herbacea, S. laggeri, S. nigri- cans (incl. ssp. alpicola), S. purpurea (incl. ssp. angustior), S. reticulata, S. retusa, S. × rubens, S. serpyllifolia, S. × smithiana, S. viminalis, S. waldsteiniana (Salicaceae). A Larix:
L. decidua (Pinaceae).
NOTES: We use the name M. laricis-epitea Kleb. (Klebahn 1899b) as circumscribed by Gm 1959. Gm 1959 distinguished six formae speciales within M. laricis-epitea that we do not list separately.
Melampsora laricis-pentandrae Kleb.
Life cycle/spore states: S AC – U T
Host(s): T Salix: S. pentandra (Salicaceae). A Larix: L. decidua (Pinaceae).
Melampsora laricis-populina Kleb.
Life cycle/spore states: S AC – U T
Host(s): T Populus: P. balsamifera, P. canadensis, P. deltoides, P. nigra (incl. var. italica), P.
cf. simonii, P. cf. yunnanensis (Salicaceae). A Larix: L. decidua (Pinaceae).