Mesenchymal stem cells: Properties and clinical potential for cell based therapies in reconstructive surgery with a focus on peripheral nerve surgery
Mesenchymale Stammzellen: Eigenschaften und klinisches Potential für zellbasierte Therapien in der rekonstruktiven Chirurgie mit dem Schwerpunkt der peripheren Nervenchirurgie
Abstract
The isolation and expansion of multipotent mesenchymal stem cells (MSCs) could be demonstrated from bone marrow, peripheral blood,
Jörn W. Kuhbier
1Kerstin Reimers
1skin, umbilical cord blood and adipose issue. They can be differentiated
Bernd Schmitz
1to different mesodermal cell lines like bone, cartilage, muscle or adipose
Peter M. Vogt
1tissue cells in vitro as well as in vivo. Thus MSCs represent an attractive cell population for the substitution of mesenchymal tissues via tissue
Christine Radtke
1engineering due to their potential of differentiation and their favourable expansion properties. In contrast to embryonic stem cells (ESCs) they
have the advantage that they can be autologously harvested in high
1 Department of Plastic, Aesthetic, Hand- andnumbers. Besides, there are fewer ethical issues in the use of MSCs.
Reconstructive Surgery,
Another advantage of MSCs is the highly regenerative secretion profile
Hannover Medical School, Hannover, Germany
of cytokines and growth factors, in particular supporting angiogenesis.
A plethora of studies describe the morphological and phenotypical characterization of this cell type as well as regulatory mechanisms underlying the differentiation into specific tissues aiming to optimize in vitro conditions for differentiation and thus clinical application.
This review describes the definition of a mesenchymal stem cell, methods for isolation and phenotypical characterization, possibilities of differentiation and possible therapeutical applications of MSCs.
Zusammenfassung
Die Isolierung und die Expansion pluripotenter mesenchymaler Stammzellen (MSCs) konnte aus Knochenmark, peripherem Blut, Haut, Nabelschnurblut und Fettgewebe demonstriert werden. Sie können sich zu verschiedenen mesodermalen Zellarten wie Knochen-, Knorpel-, Muskel- oder Fettzellen sowohl in vitro wie auch in vivo differenzieren.
Somit stellen die MSCs aufgrund ihres mesenchymalen Differenzierungs-
potentials und ihrer guten in vitro-Expansionseigenschaften eine attrak-
tive Zellpopulation für den Ersatz mesenchymaler Gewebe für das Tissue
Engineering (TE) dar. Im Gegensatz zu embryonalen Stammzellen (ESCs)
haben sie den Vorteil, dass sie in ausreichend hoher Zellzahl autolog
gewonnen werden können. Darüber hinaus bestehen bei den MSCs
keine ethischen Problemstellungen. Ein weiterer Vorteil von mesenchy-
malen Stammzellen ist ihr hochgradig regeneratives, insbesondere
Angiogenese förderndes Sekretionsprofil von Zytokinen und Wachstums-
faktoren. Eine Vielzahl von Studien beschreibt die morphologische und
phänotypische Charakterisierung dieser Zellart und Regulationsmecha-
nismen bei der Differenzierung in bestimmte Gewebespezifikationen
mit dem Ziel, die in vitro-Konditionen zur Differenzierung zu optimieren
und um somit die klinische Anwendung zu erleichtern.
Dieser Übersichtsartikel beschreibt die Definition der mesenchymalen Stammzellen, die Methoden zur Isolierung und phänotypischen Charak- terisierungen, die Differenzierungsmöglichkeiten und mögliche thera- peutische Anwendungen der MSCs.
Introduction
More than 1.5 million patients in Germany were treated because of degenerative diseases in 2002 [1]. However, many more patients remain untreated aside from the significant demographic change towards an older popu- lation in all western countries. Thus, it is expected that the number of U.S. citizens older than 65 years will double during the next twenty years while the number of citizens older than 85 years will quadruplicate.
Likewise the number of patients in need of treatment because of degenerative diseases will rise. The demand of soft tissue substitute is equally high as a number of 5.6 million patients in the USA alone underwent recon- structive surgery due to trauma or tumor resections in 2012 [2].
In this context, the subcutaneous adipose tissue plays a central role and is the crucial component in reconstructive surgery to restore the superficial surface and shape in an aesthetic fashion [3], [4], [5], [6], [7], [8], [9].
With that being said, there is a need for new therapeutic approaches because autologous transplantations tend to lose up to 40–60% of the original volume [9], [10]. Al- logenic, xenogenic or synthetic materials may cause ad- verse immunological reactions [11], [12]. Considering this, the use of stem cells may be advantageous for autologous tissue engineering as well as cell-based therapies which aim to stimulate endogenous regenera- tive capacities.
The primary purpose of transplanting adult stem cells of different origins is to repair and regenerate the resident tissue after injuries. For this task, the self-renewal prop- erties of stem cells, i.e. division to one daughter cell that will differentiate to a mature, terminally differentiated cell and one new adult stem cell, are a prerequisite. The former, so-called progenitor cell will further divide to build complexes of mature cells while the latter serves as a cell “reserve” for self-renewal.
These stem cells are resident in different tissues, but bone marrow is a renowned source for stem cells.
Derived from the embryonal mesoderm, bone marrow contains two populations of adult stem cells. One popu- lation is the hematopoietic stem cell (HSC) which is re- sponsible for all mature cell lines in peripheral blood.
There is a well-characterized population of cells capable of self-renewal, which produce progenitor cells and are able to differentiate into mature blood cells.
Bone marrow also contains mesenchymal stem or stroma cells (MSCs) which can influence hematopoietic differen- tiation via secretion of cytokines and growth factors.
Likewise, these MSCs are able to differentiate into osteo- genic, chondrogenic, adpiogenic, myogenic and neurogen- ic lineages, i.e. they are capable of transdifferentiation [13].
Despite the recognition of MSCs as stem cells due to their capability of self-renewal and differentiation into different cell lines, it is yet unknown to what extent MSCs are re- sponsible for normal growth and preservation of tissues.
Recent studies raise promising expectations on the po- tential of theses stem cells for future cell-based therapies.
However, in spite of many results it remains challenging to establish a consistent and standardized definition of this type of stem cell. There are different protocols exist- ing for the isolation, identification and differentiation of MSCs.
Definition of stem cells
There are differences between toti- and multipotent stem cells. Zygote embryonic stem cells are totipotent while adult stem cells are multipotent [14], [15]. Totipotency describes the ability to transdifferentiate, i.e. the devel- opment of cells from all three germ layers that is meso- dermal, ectodermal and endodermal differentiation.
Multipotency means the potential to differentiate within one germ layer. Adult multipotent stem cells exist in many tissues, for example in skeletal muscle, in adipose tissue or in bone marrow [16].
The totipotent embryonic stem cells were established in 1998 by Shamblott et al. [17] and Thomson et al. [18].
Despite the particular scientific significance, the applica- tion of this cell population is restricted by ethical and legal reasons. Additionally, histocompatibility or tenden- cies towards degeneracy are not yet clarified.
During isolation of MSCs from different tissues at first a heterogeneous mixture of cells is obtained from which MSCs can be filtered by application of these definitions.
In a position paper from the International Society of Cel- lular Therapy from the year 2006 it is stated that MSCs have the following properties [19]:
1. Adherence on plastic surfaces under standard cell culture conditions
2. Specific phenotype of surface antigens:
Positivity (>95% positive cells) for CD73, CD90 and CD105
Negativity (<2% positive cells) for CD45, CD34, HLA- DR as well as CD14 or CD11b and CD79α or CD19 3. In vitro differentiation in osteogenic, adipogenic or
chondrogenic cell line induced by supplements to cell
culture media (verified by specific histological
stainings)
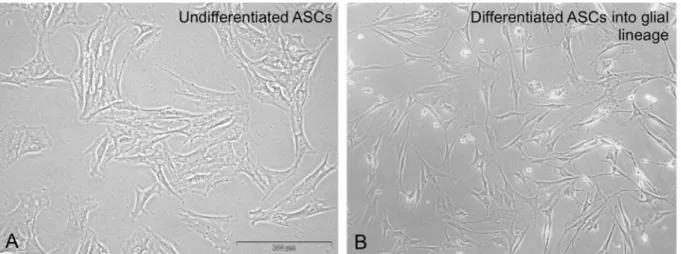
Figure 1: Light microscopy of undifferentiated ASCs in culture (A) and after differentiation into glia cells with characteristic bipolar- shape morphology. Scale bar in (A) represents 300 µm and pertains to (A) and (B)
Adult mesenchymal stem cells
In 1867 the pathologist Cohnheim suggested for the first time the existence of non-hematopoietic adult stem cells.
In the context of wound healing studies he injected a dye into the blood of testing animals to investigate to what extent dyed cells might be found in the wound. Beside inflammatory cells, which were assigned to the hematopoi- etic system, he found cells with a fibroblast-like morphol- ogy. Accordingly, Cohnheim deducted that the bone marrow has to be a reserve of such cells. In 1976, Friedenstein was successful in isolating the cell type de- scribed by Cohnheim. Friedenstein cultivated bone mar- row cells on untreated culture dishes and waited for 4 hours before he rinsed non-adherent cells. In the culture dishes cells could be observed which were strongly adher- ent and showed a heterogenous morphology. This spindle- shaped cell type formed colonies containing three to four cells. The cell cultures remained inactive for two to four days before an extreme proliferation occurred. In following studies it was shown that this extensive growth is depend- ent on the method of isolation, the density of these stem cells in the bone marrow as well as age and general health condition of the donor [20], [21], [22], [23].
The heterogeneity of the MSC cultures observed by Friedenstein could be confirmed by Conget and Minguell [24]. In that article, it was stated that there is not just one but three different cell types which can be distin- guished:
1. a proliferative-active, big, flat cell,
2. a proliferative-active, spindle-shaped cell and 3. a small, round, self-perpetuating cell.
Colter et al. verified this observation and characterized the different cell types in MSC cultures more precisely [25]. A small amount in the cultures formed the so-called RS-1 cells which display a slender, agranular cellular morphology. Furthermore, those cells have a limited ca- pacity for colony-forming and are negative for Ki-67, a cell-cycle antigen and marker of proliferation. An unprolif- erative cell phenotype is indicated by a slight content of DNA as well as an increased expression of the ornithine-
decarboxylase antizyme [26]. Beside this RS-1 cells, there exists a population of the so-called mMSCs which show a high proliferation rate.
Differentiation potential of adult stem cells
The differentiation potential of adult stem cells was de- scribed at first by the so called predestination theory.
This theory said that tissue- or organ-specific stem cells can just differentiate to terminal cell lines of this potential tissue or organ, respectively. Following this theory, bone marrow stem cells can just differentiate in hematopoetic lines and stem cells derived from the brain into neurogen- ic lines [27], [28]. Regarding BM-MSCs this theory was advanced by Haynesworth et al. [29], Prockop [30] and Pittenger et al. [15]. Those groups could prove that BM- MSCs have the potential to differentiate into mesen- chymal and non-mesenchymal cell lines. BM-MSCs can differentiate into osteogenic [31], [32], [33], chondrogenic [15], [34], [35], adipogenic [15] and myogenic lines [15], [30], [36], [37]. Furthermore, a differentiation to neural tissue [36], [38], [39], [40], [41] and tendon tissue [42], [43] could be induced. The exact mechanism of differen- tiation is as yet unresolved, particularly with regard to neural differentiation [44], [45]. In contrast, for bone marrow-derived MSCs (BM-MSCs) it can be deduced that they can be differentiated into adipogeic, chondrogenic and osteogenic cell lines as e.g. bone itself is steadily remodelling and as necessary in partial recovery e.g. after a fracture [46].
Another, often investigated cell population of MSCs are the so called adipose-derived stem cells (ASCs; Figure 1).
Like BM-MSCs these ASCs have the potential for mesen- chymal and non-mesenchymal differentiation [15]. Hence ASCs could be differentiated into adipocytes, chondro- cytes, osteoblasts, myocytes [47], endothelial cells [48], neuron-like cells [49], [50], [51], hepatocytes [52], [53], pancreatic cells [54] and hematopoietic cells [55].
ASCs offer the advantage to be less traumatic in their
extraction with higher yields.
Moreover adipose tissue accumulates as waste product after liposuctions or dermolipectomies in plastic surgery with the result that here exists a big reservoir for plastic surgical research. Most favourable, stem cells can be isolated in high amounts from adipose tissue – from 1 gram of adipose tissue, 5×10
3ASCs can be isolated, i.e. the 500-fold amount than from 1 gram of bone mar- row [56].
Characterization of MSCs
Beside the multipotent differentiation capability, MSCs from the bone marrow offer other positive properties. In general, they are easy to isolate and cultivate, thereby exhibiting a great potential of proliferation; from a single MSC, up to 7×10
7MSCs can originate until passage 6 and up to 5,5×10
8to 1,2×10
9until passage 10–25 [24], [57]. Moreover, MSCs are not immunocompetent which renders them attractive for allogenic transplantations – no immune response could be seen after MSC transplan- tation between immunocompatible patients in a study by LeBlanc [58].
Collagen type I, type II, fibronectin and laminin could be identified in the extracellular matrix of MSCs [59]. Further- more, interleukins (IL) -6, IL-7, IL-8, IL-11, IL-12, IL-14, IL- 15 and the factors macrophage-colony stimulating factor (M-CSF), leukemia inhibitory factor (LIF) and Skp, Cullin, F-box containing complex (SCF) are secreted. Additionally, MSCs secrete IL-1α, LIF, granulocyte-colony stimulating factor (G-SCF) and granulocyte macrophage-colony stimulating factor [60], [61], [62].
MSCs are not only influenced by the prevailing microen- vironment, but do influence the microenvironment as well [63]. It was shown that MSCs influence hematopoiesis [64], [65]. A significant issue is the so-called stem cell niche, i.e. a specific microenvironment with corresponding contacts between cells and extracellular matrix which is considered in recent publications as a determinant con- cerning the question whether a cell with unlimited cap- acity of self renewal is a stem cell or a cancer cell [66], [67].
BM-MSC as well as ASCs express the marker STRO-1 which is a marker of multilineage progenitor cells from the bone marrow [68], [69]. Zuk et al. could find two markers by which a distinction between the two ASCs and BM-MSCs is possible: CD 49d (α4 integrin) and CD 106 (vascular cell adhesion molecule; VCAM). ASCs are posi- tive for CD 49d and BM-MSCs are not, and vice versa for the marker CD106 [70].
Future therapy alternatives
From a therapeutical point of view, MSCs allow attractive approaches. Kopen et al. injected MSCs in the lateral ventricle of mice [41]. It could be observed that those cells were integrated into brain tissue, adopted the mor- phology of astrocytes and expressed glial fibrillary acidic
protein (GFAP). In some cells, there was even expression of the protein neuronal nuclei (NeuN), indicating a neur- onal differentiation [41]. Beside this systemic or local transplantation, the use of MSCs in genetic therapy was suggested. Keating et al. showed that MSCs, which were transfected with the gene for factor IX and implanted in vivo, secreted this factor for certain time periods – which outlines an interesting therapeutical approach for hemo- philia B [59]. Based on the therapy models presented herein, approaches for several diseases could be de- veloped, for example for facial nerve repair [71], [72], osteogenesis imperfecta [73], [74], degenerative arthritis [75] or multiple sclerosis [76].
Tissue engineering
Tissue engineering (TE) displays an alternative therapy in this context. If a patient needs repair of a long nerve defect (>3 cm), there are different methods possible:
1. Autologous nerve transplantation, for example the sural nerve
2. Allogenic nerve transplantation
3. Implantation of an artificial biocompatible nerve con- duit
However, there are certain limitations to these strategies.
Autologous nerve transplants show pathological changes in many cases and are available only in limited amounts.
Allogenic nerve transplants can lead to immune re- sponses in terms of host-versus-graft reaction. Artificial biocompatible nerve conduits in contrast are prone to loosen, to rupture, to dislocate or to interact with the surrounding tissue in terms of regeneration-inhibiting scar tissue [9]. TE aims to preserve functionally active cells that support development of new tissue inside a biological scaffold by secretion of growth factors [57], [76], [77], [78], [79], [80], [81], [82].
The basic principles of TE can be described as follows:
1. Healthy cells are required that a.) are not immunogenic, b.) can be isolated easily,
c.) show a high proliferative capacity, i.e. are respon- sible to external stimuli (for example growth factors).
2. There have to be carriers existing that can support cell differentiation in vitro and can be transplanted afterwards.
3. Bioactive molecules are required that are able to in- duce and control cell differentiation and maturation [83], [84], [85].
Regarding this, stem cells as such are of particular in-
terest for TE purposes as these cells own the capability
for unlimited self-renewal and may undergo differentiation
processes [86], [87]. It can be stated that within the
scope of TE the ideal cell should be immunocompatible,
self-perpetuating and capable of differenitiation or
transdifferentiation, respectively. Moreover, the ideal
source or resort should be easy to access, be available
Table 1: Secretion of growth factors by MSCs (for VEGF additional measurement in normal (basal) and hypoxic conditions) [88]
to a great extent and be dispensable without limitation of function of the donor organism. Regarding these re- quirements for cell and tissue for TE, the advantages of ASC in contrast to BM-MSC become apparent.
Regenerative potential of mesenchymal stem cells
Beside the direct advantages of stem cells as cell popu- lations used for TE, MSCs display a highly regenerative secretion profile. The growth factors released by MSCs are highly angiogenic, the values for some of the most significant are depicted in Table 1 [88]. Considerably, the secretion of angiogenic growth factors increases substan- tially if ASCs are cultured under hypoxic conditions. For example, the value for vascular endothelial growth factor (VEGF) raises under hypoxic conditions up to the 5-fold of the value under normal conditions [88].
Stem cells for nerve regeneration
Experimental cell transplantations were utilized success- fully to support axonal regeneration of spinal cords axons that usually have minimal regenerative properties [89], [90].
In these studies satisfying functional results could be obtained, for that reason such therapies seem promising for clinical approaches [91], [92].
Thereby, MSC-based transplantations as supplement for nerve reconstruction were considered for several reasons:
These cells support the guidance of the outgrowing axons and deliver trophic support for the spinal cord which en- ables spinal cord axons to regenerate. To combine nerve repair with the additive application of myelin-forming cells is a relatively simple, fast and efficient method to support peripheral nerve regeneration as well [93], [94], [95], [96], [97].
Substantial issues are released neurotrophic growth factors like nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF) as well as Neurotropin-3 and -4 (NT-3 and -4) [98], [99].
For functional nerve regeneration, not only axonal sprouting and elongation of neurites are necessary but also a remyelination with adequate expression of ion channels in the region of Ranvier’s nodes to restore ap-
propriate nerve conduction velocity. Consequently, remyelination is a prerequisite for recovery of normal nerve conduction velocity on the one hand and protection of axons against degenerative processes on the other hand.
If in an acute nerve lesion myelin-forming cells are transplanted, they are already activated for myelin forming at the point of transplantation while resident Schwann cells have to be activated in a signal cascade. Moreover, damaged spinal dorsal root nerve cells are protected by MSCs from apoptosis [100].
In different studies, glial as well as neuronal differenti- ation of ASCs could be shown in vitro ([101], [102], [103], [104], [105], [106], [107], Figure 1A.
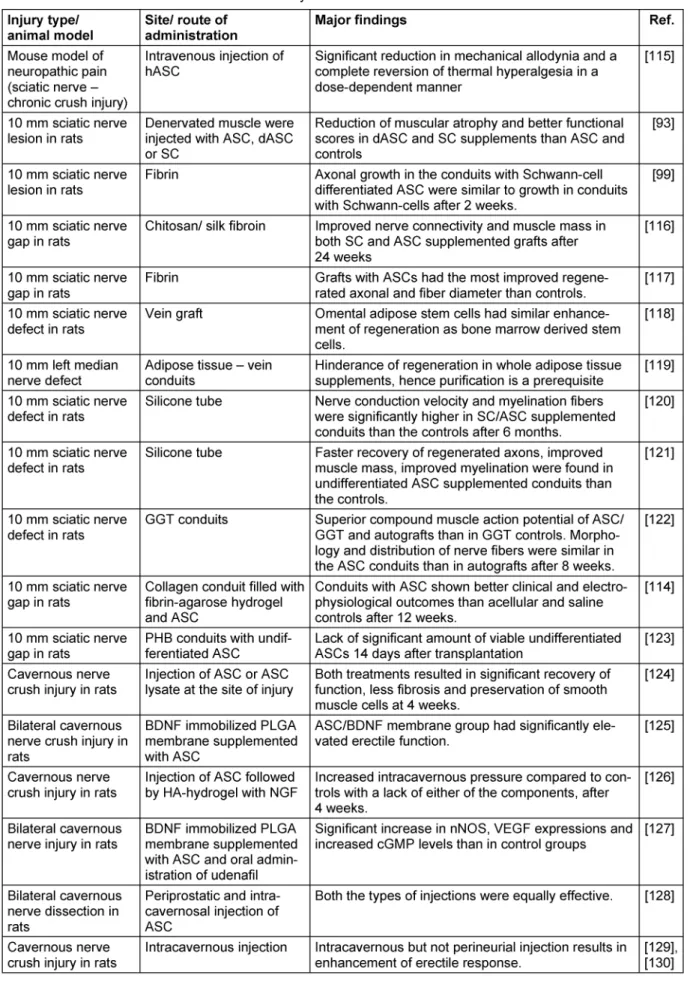
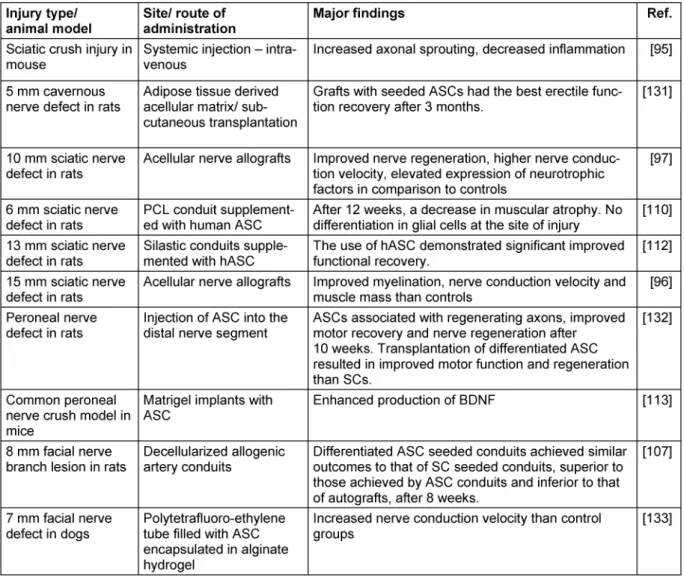
By induction with FGF and EGF, ASCs developed typical morphological and phenotypical characteristics of neurospheres while subsequent deprivation of both resulted in glial differentiation [108], [109]. However, when administered at the site of nerve injury in vivo, they remain undifferentiated and do not differentiate into neuronal cells [110], [111], [112]. They enhance peripher- al nerve regeneration by secretion of BDNF, NGF and FGF and also prevent dorsal root ganglia neurons from under- going apoptosis [113], [100]. In multiple studies, their regenerative effect on peripheral nerve injuries could be shown either by administration of ASCs either locally by injection or on a tubular or scaffold or systemic [93], [95], [96], [97], [99], [107], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133]. For more detailed information, see Table 2.
These results implicate innovative therapy strategies for the treatment of demyelinating diseases as well as trau- matic nerve lesions. The advantage of neuronal and glial differentiation of ASCs regarding clinical application is the relatively simple and less invasive isolation of ASCs compared to the more invasive isolation of bone marrow.
Preliminary works depict that direct as well as intravenous
transplantation of MSCs in lesions of the spinal cord
result in a “homing” of these cells into the lesion and lead
to a considerable reparation in terms of axonal regenera-
tion and remyelination in the central nervous system
[115], [134]. The combination of surgical nerve suture
and transplantation of myelin-forming cells that support
axonal regeneration and remyelinate demyelinated axons
displayed a significant advantage compared to the control
groups without cell transplantation [135].
Table 2: Summary of in vivo-studies on the applications of adipose derived stem cells in the therapy of peripheral nervous system disorders
(Continued)
Table 2: Summary of in vivo-studies on the applications of adipose derived stem cells in the therapy of peripheral nervous system disorders
Conclusions
Adult stem cells from adipose tissue not only have the capability of mesodermal differentiation but also the po- tential of transdifferentiation.
These ASCs meet all criteria that are of essential import- ance for TE and thus have a great potential for regenera- tive medicine. They are easy to harvest in plastic surgical standard procedures, which makes adipose tissue an attractive source for these multipotent cells.
Furthermore, other possible applications with promising
results exist via cell-based therapies in terms of trans-
plantation of MSCs due to their most favourable secretion
profile of cytokines and trophic factors. Positive results
could be achieved by injecting ASCs to the local site of
injury, however, intravenous injection seems also to be
promising. Considering this, ASCs might play a substantial
role in future regenerative medicine, in particular concern-
ing nerve regeneration approaches.
List of abbreviations
ASCs – adipose-derived stem cells BDNF – brain-derived neurotrophic factor bFGF – basic fibroblast growth factor
BM-MSCs – bone marrow-derived mesenchymal stem cells
CD – cluster of differentiation dASC – differentiated ASC EGF – epithelial growth factor FGF – fibroblast growth factor
GDNF – glial-derived neurotrophic factor GFAP – glial fibrillary acidic protein
GGT – genipin-gelatin-tricalcium phosphate
GM-CSF – granulocyte/macrophage-colony stimulating factor
G-CSF – granulocyte-colony stimulating factor HA hydrogel – hydroxyapatite hydrogel hASC – human ASC
HGF – hepatocyte growth factor HSCs – hematopoietic stem cells IL – interleukin
LIF – leukemia inhibitory factor
MCAO – middle cerebral artery occlusion M-CSF – macrophage-colony stimulating factor MSCs – mesenchymal stem cells
NGF – nerve growth factor NeuN – neuronal nuclei
nNOS – neuronal nitric oxide synthase NT – neurotropin
PCL – nolycaprolacton
PLGA – poly(lactic-co-glycolic acid) SC – Schwann cells
SCF – Skp, Cullin, F-box containing complex TE – tissue engineering
TGF-β – transforming growth factor β VEGF – vascular endothelial growth factor
Notes
Acknowledgement
This work has been supported by the Boehringer Ingel- heim Foundation. We thank Sankaranarayanan Sivakmar for support in literature review.
Competing interests
The authors declare that they have no competing in- terests.
References
1. Ringe J, Kaps C, Schmitt B, Büscher K, Bartel J, Smolian H, Schultz O, Burmester GR, Häupl T, Sittinger M. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002 Mar;307(3):321-7. DOI:
10.1007/s00441-002-0525-z
2. American Society of Plastic Surgeons, editor. 2012 Plastic Surgery Statistics Report. ASPS; 2013 [cited 12.10.2013].
Available from: http://www.plasticsurgery.org/Documents/news- resources/statistics/2012-Plastic-Surgery-Statistics/full-plastic- surgery-statistics-report.pdf
3. Coleman SR. Structural fat grafting: more than a permanent filler.
Plast Reconstr Surg. 2006 Sep;118(3 Suppl):108S-120S. DOI:
10.1097/01.prs.0000234610.81672.e7
4. Beahm EK, Walton RL, Patrick CW Jr. Progress in adipose tissue construct development. Clin Plast Surg. 2003 Oct;30(4):547-58, viii. DOI: 10.1016/S0094-1298(03)00072-5
5. Alster TS, West TB. Human-derived and new synthetic injectable materials for soft-tissue augmentation: current status and role in cosmetic surgery. Plast Reconstr Surg. 2000 Jun;105(7):2515- 25; discussion 2526-8. DOI: 10.1097/00006534-200006000- 00034
6. Brey EM, Patrick CW Jr. Tissue engineering applied to reconstructive surgery. IEEE Eng Med Biol Mag. 2000 Sep- Oct;19(5):122-5. DOI: 10.1109/51.870241
7. Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999 Oct;26(4):587-603, viii.
8. Patrick CW Jr. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001 Aug;263(4):361-6. DOI: 10.1002/ar.1113 9. Patrick CW Jr, Chauvin PB, Hobley J, Reece GP. Preadipocyte
seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999 Apr;5(2):139-51. DOI: 10.1089/ten.1999.5.139 10. Patrick CW Jr, Zheng B, Johnston C, Reece GP. Long-term
implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng.
2002 Apr;8(2):283-93. DOI: 10.1089/107632702753725049 11. Butler DL, Awad HA. Perspectives on cell and collagen composites
for tendon repair. Clin Orthop Relat Res. 1999 Oct;(367 Suppl):S324-32.
12. Eppley BL. Alloplastic implantation. Plast Reconstr Surg. 1999 Nov;104(6):1761-83. DOI: 10.1097/00006534-199911000- 00025
13. Kuhbier JW, Weyand B, Radtke C, Vogt PM, Kasper C, Reimers K. Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol.
2010;123:55-105. DOI: 10.1007/10_2009_24 14. Gage FH. Mammalian neural stem cells. Science. 2000
Feb;287(5457):1433-8. DOI: 10.1126/science.287.5457.1433 15. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca
JD, Moorman MA, Simonetti DW, Craig S, Marshak DR.
Multilineage potential of adult human mesenchymal stem cells.
Science. 1999 Apr;284(5411):143-7. DOI:
10.1126/science.284.5411.143
16. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004 Apr;36(4):568-84. DOI:
10.1016/j.biocel.2003.11.001
17. Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998 Nov;95(23):13726-31. DOI:
10.1073/pnas.95.23.13726
18. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov;282(5391):1145-7. DOI:
10.1126/science.282.5391.1145
19. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM.
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. DOI:
10.1080/14653240600855905
20. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999 Nov;107(2):275-81. DOI: 10.1046/j.1365- 2141.1999.01715.x
21. Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupob A, Canceddaa R, Quartoa R. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999 Sep;27(9):1460-6. DOI:
10.1016/S0301-472X(99)00076-4
22. Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self- renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997 Feb;64(2):278- 94. DOI: 10.1002/(SICI)1097-4644(199702)64:2<278::AID- JCB11>3.0.CO;2-F
23. Caplan AI. The mesengenic process. Clin Plast Surg. 1994 Jul;21(3):429-35.
24. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999 Oct;181(1):67-73. DOI: 10.1002/(SICI)1097- 4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C
25. Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000
Mar;97(7):3213-8. DOI: 10.1073/pnas.070034097; DOI:
10.1073/pnas.97.7.3213
26. Iwata S, Sato Y, Asada M, Takagi M, Tsujimoto A, Inaba T, Yamada T, Sakamoto S, Yata J, Shimogori T, Igarashi K, Mizutani S. Anti- tumor activity of antizyme which targets the ornithine
decarboxylase (ODC) required for cell growth and transformation.
Oncogene. 1999 Jan;18(1):165-72. DOI:
10.1038/sj.onc.1202275
27. McKay R. Stem cells in the central nervous system. Science.
1997 Apr;276(5309):66-71. DOI: 10.1126/science.276.5309.66 28. Sachs L. The molecular control of blood cell development.
Science. 1987 Dec;238(4832):1374-9. DOI:
10.1126/science.3317831
29. Haynesworth SE, Barer MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13(1):69-80. DOI:
10.1016/8756-3282(92)90363-2
30. Prockop DJ. Marrow stromal cells as stem cells for
nonhematopoietic tissues. Science. 1997 Apr;276(5309):71-4.
DOI: 10.1126/science.276.5309.71
31. Long MW. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001 May-Jun;27(3):677-90. DOI:
10.1006/bcmd.2001.0431
32. Karsenty G. Bone formation and factors affecting this process.
Matrix Biol. 2000 May;19(2):85-9. DOI: 10.1016/S0945- 053X(00)00053-6
33. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995 Aug;17(2)Supplement 1:S77-S83.
DOI: 10.1016/8756-3282(95)00183-E
34. Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow:
differentiation-dependent gene expression of matrix components.
Exp Cell Res. 2001 Aug;268(2):189-200. DOI:
10.1006/excr.2001.5278
35. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998 Jan;238(1):265-72. DOI:
10.1006/excr.1997.3858
36. Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells.
Ann N Y Acad Sci. 2001Jun;938:231-5. DOI: 10.1111/j.1749- 6632.2001.tb03593.x
37. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine.
Muscle Nerve. 1995 Dec;18(12):1417-26. DOI:
10.1002/mus.880181212
38. Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A, Bunnell BA. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004 Aug;117:4289-99. DOI: 10.1242/jcs.01264 39. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford
T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000 Aug;164(2):247-56.
DOI: 10.1006/exnr.2000.7389
40. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000 Aug;61(4):364-70. DOI: 10.1002/1097- 4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C
41. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999 Sep;96(19):10711-6. DOI:
10.1073/pnas.96.19.10711
42. Awad HA, Butler DL, Boivin GP, Smith FNL, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999 Jun;5(3):267-77. DOI:
10.1089/ten.1999.5.267
43. Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ.
Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998 Jul;16(4):406-13. DOI:
10.1002/jor.1100160403
44. Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005 Nov;205(2):194-201. DOI: 10.1002/jcp.20376
45. Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I.
Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004 Jul;77(2):192-204. DOI: 10.1002/jnr.20147 46. Hall BK, Miyake T. Divide, accumulate, differentiate: cell
condensation in skeletal development revisited. Int J Dev Biol.
1995 Dec;39(6):881-93.
47. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211-28. DOI: 10.1089/107632701300062859
48. Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004; 109:656-63. DOI:
10.1161/01.CIR.0000114522.38265.61
49. Fujimura J, Ogawa R, Mizuno H, Fukunaga Y, Suzuki H. Neural differentiation of adipose-derived stem cells isolated from GFP transgenic mice. Biochem Biophys Res Commun. 2005 Jul;333(1):116-21. DOI: 10.1016/j.bbrc.2005.05.096 50. Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk
PA, Lorenz HP, Benhaim P, Hedrick MH. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003 May;111(6):1922-31.
DOI: 10.1097/01.PRS.0000055043.62589.05
51. Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002 Jun;294(2):371-9. DOI: 10.1016/S0006- 291X(02)00469-2
52. Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo.
Biochem Biophys Res Commun. 2005 Mar;328(1):258-64. DOI:
10.1016/j.bbrc.2004.12.158
53. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007 Jul;46(1):219-28. DOI: 10.1002/hep.21704
54. Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun.
2006 Mar;341(4):1135-40. DOI: 10.1016/j.bbrc.2006.01.072 55. Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G,
Penicaud L, Casteilla L, Laharrague P. Human subcutaneous adipose cells support complete differentiation but not self- renewal of hematopoietic progenitors. J Cell Physiol. 2006 Aug;208(2):282-8. DOI: 10.1002/jcp.20655
56. Kitagawa Y, Korobi M, Toriyama K, Kamei Y, Torii S. History of discovery of human adipose-derived stem cells and their clinical application. Jpn J Plast Reconstr Surg. 2006; 49:1097-1104.
57. Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004 Jul;32(7):911-23.
58. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus- host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004 May;363(9419):1439-41. DOI:
10.1016/S0140-6736(04)16104-7
59. Keating A, Horsfall W, Hawley RG, Toneguzzo F. Effect of different promoters on expression of genes introduced into hematopoietic and marrow stromal cells by electroporation. Exp Hematol. 1990 Feb;18(2):99-102.
60. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000 Aug;28(8):875-84.
DOI: 10.1016/S0301-472X(00)00482-3
61. Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL.
Phenotypic and functional comparison of cultures of marrow- derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998 Jul;176(1):57-66. DOI: 10.1002/(SICI)1097- 4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7
62. Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro:
effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996 Mar;166(3):585-92. DOI: 10.1002/(SICI)1097-
4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6
63. Wieczorek G, Steinhoff C, Schulz R, Scheller M, Vingron M, Ropers HH, Nuber UA. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res. 2003 Feb;311(2):227-37.
64. Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000 Dec;9(6):841-8. DOI: 10.1089/152581600750062264 65. McManus PM, Weiss L. Busulfan-induced chronic bone marrow
failure: changes in cortical bone, marrow stromal cells, and adherent cell colonies. Blood. 1984 Nov;64(5):1036-41.
66. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010 Feb;222(2):268-77. DOI:
10.1002/jcp.21940
67. Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006 May;66(9):4553-7. DOI:
10.1158/0008-5472.CAN-05-3986
68. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+
marrow cell population is multipotential. Cells Tissues Organs.
2002;170(2-3):73-82. DOI: 10.1159/000046182 69. Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+
fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994 Dec 15;84(12):4164-73.
70. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002 Dec;13(12):4279-95. DOI: 10.1091/mbc.E02-02-0105 71. Baumgartner BJ, Shine HD. Permanent rescue of lesioned
neonatal motoneurons and enhanced axonal regeneration by adenovirus-mediated expression of glial cell-line-derived neurotrophic factor. J Neurosci Res. 1998 Dec;54(6):766-77.
DOI: 10.1002/(SICI)1097-4547(19981215)54:6<766::AID- JNR4>3.0.CO;2-A
72. Baumgartner BJ, Shine HD. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci. 1997 Sep;17(17):6504-11.
73. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK.
Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta.
Nat Med. 1999 Mar;5(3):309-13. DOI: 10.1038/6529 74. Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class
R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta.
Proc Natl Acad Sci USA. 1998 Feb;95(3):1142-7. DOI:
10.1073/pnas.95.3.1142
75. Caplan AI. Stem cell delivery vehicle. Biomaterials. 1990 Jul;11:44-6.
76. Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human- shaped mandibular condyle from rat mesenchymal stem cells.
J Dent Res. 2003 Dec;82(12):951-6. DOI:
10.1177/154405910308201203
77. Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002 Nov;23(22):4315-23. DOI:
10.1016/S0142-9612(02)00176-X
78. Fu J, Fiegel J, Krauland E, Hanes J. New polymeric carriers for controlled drug delivery following inhalation or injection.
Biomaterials. 2002 Nov;23(22):4425-33. DOI: 10.1016/S0142- 9612(02)00182-5
79. Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002 Nov;23(22):4307- 14. DOI: 10.1016/S0142-9612(02)00175-8
80. Poshusta AK, Anseth KS. Photopolymerized biomaterials for application in the temporomandibular joint. Cells Tissues Organs.
2001;169:272-8. DOI: 10.1159/000047891
81. Sittinger M, Bujia J, Rotter N, Reitzel D, Minuth WW, Burmester GR. Tissue engineering and autologous transplant formation:
practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials. 1996;17(3):237-42. DOI:
10.1016/0142-9612(96)85561-X
82. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells.
J Neurosci. 2002 Aug;22(15):6623-30.
83. Halberstadt C, Austin C, Rowley J, Culberson C, Loebsack A, Wyatt S, Coleman S, Blacksten L, Burg K, Mooney D, Holder W Jr. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Eng.
2002 Apr;8(2):309-19. DOI: 10.1089/107632702753725067 84. von Heimburg D, Zachariah S, Heschel I, Kühling H, Schoof H,
Hafemann B, Pallua N. Human preadipocytes seeded on freeze- dried collagen scaffolds investigated in vitro and in vivo.
Biomaterials. 2001;22:429-438. DOI: 10.1016/S0142- 9612(00)00186-1
85. Marler JJ, Guha A, Rowley J, Koka R, Mooney D, Upton J, Vacanti JP. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plast Reconstr Surg. 2000
May;105(6):2049-58. DOI: 10.1097/00006534-200005000- 00020
86. Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989 Aug;106(4):619-33.
87. Watt FM, Hogan BL. Out of Eden: stem cells and their niches.
Science. 2000 Feb;287(5457):1427-30. DOI:
10.1126/science.287.5457.1427
88. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL.
Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004 Mar;109(10):1292-8.
DOI: 10.1161/01.CIR.0000121425.42966.F1
89. Ryu HH, Kang BJ, Park SS, Kim Y, Sung GJ, Woo HM, Kim WH, Kweon OK. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012 Dec;74(12):1617-30. DOI: 10.1292/jvms.12-0065
90. Ozdemir M, Attar A, Kuzu I, Ayten M, Ozgencil E, Bozkurt M, Dalva K, Uckan D, Kılıc E, Sancak T, Kanpolat Y, Beksac M. Stem cell therapy in spinal cord injury: in vivo and postmortem tracking of bone marrow mononuclear or mesenchymal stem cells. Stem Cell Rev. 2012 Sep;8(3):953-62. DOI: 10.1007/s12015-012- 9376-5
91. Choi JS, Leem JW, Lee KH, Kim SS, Suh-Kim H, Jung SJ, Kim UJ, Lee BH. Effects of human mesenchymal stem cell transplantation combined with polymer on functional recovery following spinal cord hemisection in rats. Korean J Physiol Pharmacol. 2012 Dec;16(6):405-11. DOI: 10.4196/kjpp.2012.16.6.405
92. Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, Yoon DH, Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp. 2007;67(1):13-22.
93. Schaakxs D, Kalbermatten DF, Raffoul W, Wiberg M, Kingham PJ. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve. 2013 May;47(5):691-701. DOI: 10.1002/mus.23662 94. Gu JH, Ji YH, Dhong ES, Kim DH, Yoon ES. Transplantation of adipose derived stem cells for peripheral nerve regeneration in sciatic nerve defects of the rat. Curr Stem Cell Res Ther.
2012;7(5):347-55. DOI: 10.2174/157488812802481463 95. Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S,
Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B.
Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012 Jun;18(11- 12):1264-72. DOI: 10.1089/ten.tea.2011.0491
96. Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, Jia H, Tong XJ. Adipose-derived stem cells promote peripheral nerve repair.
Arch Med Sci. 2011 Aug;7(4):592-6. DOI:
10.5114/aoms.2011.24127
97. Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011 Oct;28(4):565-72. DOI:
10.3892/ijmm.2011.725
98. Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WEB.
Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011 Feb;29(2):169-78. DOI: 10.1002/stem.570
99. di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010 Sep;63(9):1544-52. DOI: 10.1016/j.bjps.2009.09.012 100. Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G, Kingham PJ.
Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011
Dec;199:515-22. DOI: 10.1016/j.neuroscience.2011.09.064 101. Faroni A, Terenghi G, Reid AJ. Adipose-derived stem cells and nerve regeneration: promises and pitfalls. Int Rev Neurobiol.
2013;108:121-36. DOI: 10.1016/B978-0-12-410499-0.00005- 8
102. Zhang HT, Liu ZL, Yao XQ, Yang ZJ, Xu RX. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy. 2012 Sep;14(10):1203-14. DOI: 10.3109/14653249.2012.711470 103. Li H, Han Z, Liu D, Zhao P, Liang S, Xu K. Autologous platelet-rich
plasma promotes neurogenic differentiation of human adipose- derived stem cells in vitro. Int J Neurosci. 2013 Mar;123(3):184- 90. DOI: 10.3109/00207454.2012.742077
104. Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K.
Glial differentiation of human adipose-derived stem cells:
implications for cell-based transplantation therapy. Neuroscience.
2013 Apr;236:55-65. DOI: 10.1016/j.neuroscience.2012.12.066 105. Tse KH, Sun M, Mantovani C, Terenghi G, Downes S, Kingham
PJ. In vitro evaluation of polyester-based scaffolds seeded with adipose derived stem cells for peripheral nerve regeneration. J Biomed Mater Res Part A. 2010 Dec;95A(3):701-8. DOI:
10.1002/jbm.a.32889
106. Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, Gao P, Hu G, Jin Y. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose- derived mesenchymal stem cells. Biomaterials. 2010 Jul;31(20):5312-24. DOI: 10.1016/j.biomaterials.2010.03.029
107. Sun F, Zhou K, Mi WJ, Qiu JH. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats.
Biomaterials. 2011 Nov;32(32):8118-28. DOI:
10.1016/j.biomaterials.2011.07.031
108. Radtke C, Schmitz B, Spies M, Kocsis JD, Vogt PM. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int J Dev Neurosci. 2009
Dec;27(8):817-23. DOI: 10.1016/j.ijdevneu.2009.08.006 109. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg
M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro.
Exp Neurol. 2007 Oct;207(2):267-74. DOI:
10.1016/j.expneurol.2007.06.029
110. Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG.
Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18(2):145-58. DOI:
10.3727/096368909788341289
111. Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng.
2006 Jul;12(7):1891-901. DOI: 10.1089/ten.2006.12.1891 112. Scholz T, Sumarto A, Krichevsky A, Evans GRD. Neuronal
differentiation of human adipose tissue-derived stem cells for peripheral nerve regeneration in vivo. Arch Surg. 2011 Jun;146(6):666-74. DOI: 10.1001/archsurg.2011.148 113. Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K,
Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE. 2011 Mar;6(3):e17899. DOI:
10.1371/journal.pone.0017899
114. Carriel V, Garrido-Gómez J, Hernández-Cortés P, Garzón I, García- García S, Sáez-Moreno JA, Del Carmen Sánchez-Quevedo M, Campos A, Alaminos M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng. 2013 Apr;10(2):026022. DOI:
10.1088/1741-2560/10/2/026022
115. Sacerdote P, Niada S, Franchi S, Arrigoni E, Rossi A, Yenagi V, de Girolamo L, Panerai AE, Brini AT. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2013 Apr;22(8):1252-63. DOI:
10.1089/scd.2012.0398
116. Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X.
Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med. 2011 Aug;22(8):1947-64. DOI:
10.1007/s10856-011-4370-z
117. di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011 May;181:278-91. DOI: 10.1016/j.neuroscience.2011.02.052 118. Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K.
Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose-derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve. 2011 Feb;43(2):157-63. DOI: 10.1002/mus.21895 119. Papalia I, Raimondo S, Ronchi G, Magaudda L, Giacobini-
Robecchi MG, Geuna S. Repairing nerve gaps by vein conduits filled with lipoaspirate-derived entire adipose tissue hinders nerve regeneration. Ann Anat. 2013 May;195(3):225-30. DOI:
10.1016/j.aanat.2012.10.012
120. Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg.
2012 May;65(5):657-64. DOI: 10.1016/j.bjps.2011.11.035 121. Mohammadi R, Azizi S, Amini K. Effects of undifferentiated
cultured omental adipose-derived stem cells on peripheral nerve regeneration. J Surg Res. 2013 Apr;180(2):e91-7. DOI:
10.1016/j.jss.2012.04.011
122. Shen CC, Yang YC, Liu BS. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J Biomed Mater Res Part A. 2012 Jan; 100A(1):48-63. DOI: 10.1002/jbm.a.33227 123. Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G,
Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010
Dec;63(12):e811-7. DOI: 10.1016/j.bjps.2010.08.013 124. Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin C-S, Lue TF.
Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010 Oct;7(10):3331-40.
DOI: 10.1111/j.1743-6109.2010.01875.x
125. Piao S, Kim IG, Lee JY, Hong SH, Kim SW, Hwang TK, Oh SH, Lee JH, Ra JC, Lee JY. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012 Aug;9(8):1968-79. DOI:
10.1111/j.1743-6109.2012.02760.x
126. Kim IG, Piao S, Lee JY, Hong SH, Hwang TK, Kim SW, Kim CS, Ra JC, Noh I, Lee JY. Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A. 2013 Jan;19(1-2):14-23. DOI:
10.1089/ten.TEA.2011.0654
127. Jeong HH, Piao S, Ha JN, Kim IG, Oh SH, Lee JH, Cho HJ, Hong SH, Kim SW, Lee JY. Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/brain-derived neurotrophic factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology. 2013 May;81(5):1108.e7-14. DOI:
10.1016/j.urology.2013.01.022
128. You D, Jang MJ, Lee J, Suh N, Jeong IG, Sohn DW, Kim SW, Ahn TY, Kim CS. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate. 2013 Feb;73(3):278-86. DOI:
10.1002/pros.22567
129. Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012 Jan;61(1):201-10. DOI: 10.1016/j.eururo.2011.07.061 130. Ying C, Yang M, Zheng X, Hu W, Wang X. Effects of intracavernous
injection of adipose-derived stem cells on cavernous nerve regeneration in a rat model. Cell Mol Neurobiol. 2013 Mar;33(2):233-40. DOI: 10.1007/s10571-012-9890-7 131. Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath
MH, Konety BR, Lue TF, Lin CS. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology. 2011 Jun;77(6):1509.e1-8. DOI:
10.1016/j.urology.2010.12.076
132. Tomita K, Madura T, Mantovani C, Terenghi G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. 2012 Jul;90(7):1392-402. DOI:
10.1002/jnr.23002
133. Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose- derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013 Mar;71(3):577-87. DOI:
10.1016/j.joms.2012.05.025
134. Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367-83. DOI:
10.1016/S0079-6123(06)61026-1
135. Matthes SM, Reimers K, Janssen I, Liebsch C, Kocsis JD, Vogt PM, Radtke C. Intravenous transplantation of mesenchymal stromal cells to enhance peripheral nerve regeneration. Biomed Res Int. 2013;2013:573169. DOI: 10.1155/2013/573169 136. Lorenz HP, Hedrick MH, Chang J, Mehrara BJ, Longaker MT. The
impact of biomolecular medicine and tissue engineering on plastic surgery in the 21st century. Plast Reconstr Surg. 2000 Jun;105(7):2467-81. DOI: 10.1097/00006534-200006000- 00027
137. Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells.
Neurology. 2001 Jun;56(12):1666-72. DOI:
10.1212/WNL.56.12.1666
Corresponding author:
Professor Christine Radtke, MD
Department of Plastic, Aesthetic, Hand- and Reconstructive Surgery, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany, Phone:
+49 (511) 532 - 8864, Fax: +49 (511) 532 - 8890 Radtke.christine@mh-hannover.de
Please cite as
Kuhbier JW, Reimers K, Schmitz B, Vogt PM, Radtke C. Mesenchymal stem cells: Properties and clinical potential for cell based therapies in reconstructive surgery with a focus on peripheral nerve surgery. GMS Ger Plast Reconstr Aesthet Surg. 2015;5:Doc04.
DOI: 10.3205/gpras000032, URN: urn:nbn:de:0183-gpras0000326
This article is freely available from
http://www.egms.de/en/journals/gpras/2015-5/gpras000032.shtml Published:2015-08-04
Copyright
©2015 Kuhbier et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 License. See license information at http://creativecommons.org/licenses/by/4.0/.
![Table 1: Secretion of growth factors by MSCs (for VEGF additional measurement in normal (basal) and hypoxic conditions) [88]](https://thumb-eu.123doks.com/thumbv2/1library_info/4838115.1628618/5.892.109.795.126.273/table-secretion-growth-factors-additional-measurement-hypoxic-conditions.webp)