DISSERT ATION
zur Erlangung des akademischen Grades
Doctor rerum naturalium (Dr. rer. nat.)
vorgelegt
der Fakultät Mathematik und Naturwissenschaften der Technischen Universität Dresden
von
M.Sc. Juliane Schott
geboren am 01.06.1986 in Dresden
Eingereicht am
Die Dissertation wurde in der Zeit von November 2010 bis Mai 2014 am Helmholtz-Zentrum Dresden-Rossendorf, Institut für Ressourcenökologie, sowie am Sachgebiet Strahlenschutz
der Technischen Universität Dresden, Zentrales Radionuklidlabor, angefertigt.
Acknowledgement ― Danksagung
Zunächst möchte ich mich bei den ehemaligen und jetzigen Direktoren des Instituts für Ressourcenökologie (ehemals Radiochemie) am Helmholtz-Zentrum Dresden-Rossendorf ― Prof. Dr. Gert Bernhard und Prof.
Dr. Thorsten Stumpf ― bedanken, unter deren Schirm ich meine Doktorarbeit anfer8gen konnte.
Mein großer Dank gilt Dr. Margret Acker, welche die Themen zu meinen bisherigen Abschlussarbeiten und nun auch der Doktorarbeit gestellt hat. Vielen Dank für deine jahrelange Unterstützung und dein Vertrauen.
Bei Dr. Astrid Barkleit, Dr. Vinzenz Brendler als auch wiederum bei Dr. Margret Acker möchte ich mich sehr herzlich für ihre außerordentlich intensive Betreuung und Unterstützung in der Bearbeitung meines Promotionsthemas bedanken. Unvergesslich bleiben mir euer unermüdlicher Einsatz in der Korrektur der Publikationen und Doktorarbeit, sowie eure zielführenden und motivierenden Gesprächsrunden. Auch bei Herrn Dr. Steffen Taut möchte mich recht herzlich für sein Engagement im Korrigieren von Schriftstücken und aufmerksames Begleiten dieser Arbeit bedanken.
Mein unendlicher Dank gilt Jérôme Kretzschmar, mit dessen Unterstützung und Expertise in NMR- Spektroskopie diese Arbeit solide aufgestellt werden konnte. Die Anwendung der NMR-Spektroskopie hat zur Aufklärung eines sehr komplexen Systems in einem hohen Maß beigetragen. Vielen herzlichen Dank für deine unablässige Unterstützung, unermüdlichen Einsatz, ansteckende Begeisterungsfähigkeit und Motivation. Ich habe viel von dir gelernt.
Bei Björn Drobot, welcher die PARAFAC durchgeführt hat, und Prof. Dr. Satoru Tsushima, welcher DFT Rechnungen ausgeführt hat, möchte ich mich sehr gern bedanken. Durch diese Methoden konnten Ergebnisse und Hypothesen gefestigt und das Gesamtbild über das zu bearbeitende System abgerundet werden. Vielen Dank.
Ein herzlicher Dank geht an die Aktinidenverbundprojektteilnehmer Dr. Sascha Eidner und Prof. Dr.
Michael U. Kumke vom Institut für Physikalische Chemie der Universität Potsdam für die Möglichkeit ihre Lasersysteme für Untersuchungen zu nutzen, die umfassende Betreuung während meines Aufenthalts, und Diskussion der Ergebnisse. In diesem Zusammenhang möchte ich mich auch bei den weiteren Mitarbeitern im Aktinidenverbundprojekt für einen regen Austausch und Diskussionsrunden bedanken.
Bei Prof. Dr. Eike Brunner und Dr. Silvia Paasch vom Institut für Bioanalytische Chemie der TU Dresden bedanke ich mich für die Durchführung der Festkörper-NMR Messungen und Diskussionen.
Folgenden weiteren Personen möchte ich für ihre Zusammenarbeit und Untersützung sehr gern danken:
- Karsten Heim (HZDR) für die Durchführung von IR Messungen,
- Stephan Weiß (HZDR) für die Betreuung in den Lichtstreuexperimenten, SEM-Probenpräparation und Unterstützung in labortechnischen Angelegenheit,
- Elfi Christalle (HZDR) für die Durchführung der SEM Messungen,
- Analytikgruppe des Insituts für Ressourcenökologie (HZDR) für unzählige ICP-MS Analysen,
- Dr. Henry Moll (HZDR) für die Einführung in das Lasersystem für TRLFS, Wartung des Lasersystems und damit verbunden einem reibungslosen Messablauf, sowie für die Betreuung der Cm-Arbeiten,
- Dr. Christoph Hennig (HZDR) und Dr. Sabrina Labs (Forschungszentrum Jülich) für die Organisation und Durchführen von XRD Messungen am DESY in Hamburg (ein Dank geht an diese Institution für die Möglichkeit zur Messung) und nachfolgende Diskussionen,
- Dr. Erica Brendler vom Institut für Analytische Chemie der TU Bergakademie Freiberg für die Zusammenarbeit und Möglichkeit NMR Messungen durchzuführen,
- dem Strahlenschutz und Technikern des Insituts für Ressourcenökologie (HZDR) für einen reibungslosen Laborablauf und -alltag, insbesondere Annette Rumpel, Heidemarie Heim, Kathrin Nebe, Christa Müller, Steffen Henke, Bernd Hiller, Stephan Weiß, Carola Eckardt, Heidrun Neubert und Sylvia Heller,
- Dr. Frank Bok (HZDR) für seine Expertise in thermodynamischen Datenbanken und Beratung,
- Dr. Juliane März (HZDR), Dr. Matthias Schmid (ehemals HZDR), Dr. Jörg Grenzer (HZDR) und Andrea Scholz (HZDR) für ihren Einsatz in der Röntgendiffraktometrie,
- dem Sekretariat des Insituts für Ressourcenökologie (HZDR), vorallem Jana Gorzitze und Claudia Kirmes, sowie des Sachgebiets Strahlenschutz (TU Dresden) Martina Kobus für organisatorische Angelegenheiten wie Dienstreisen und Abrechnungen, und Ronny Berndt in IT Angelegenheiten,
- Mitarbeiter des Sachgebiets Strahlenschutz der TU Dresden (Dr. Steffen Taut, Dr. Margret Acker, Franziska Taube, Melanie Müller, Norman Kelly, Martina Kobus, Wolfgang Krause, Sandra Hertwig) für ein angenehmes Arbeitsklima, und alle nicht namentlich genannten IRE-Kollegen für eine angenehme Zeit.
- Freunde und Kollegen des Büros P254 am Institut für Ressourcenökologie (HZDR) Isabel Zirnstein, Corinna Gagell, Katja Schulz, Ulrike Gerber, Siriwan Dulnee, Laura Lütke, Anne Heller, Erik Johnstone, Claudia Joseph, Claudia Wilke, sowie Jérôme Kretzschmar, Constanze Richter und Frank Bok, meinen lieben Freunden Franziska und Stephan, Maria, Madlen und René, für den guten Zusammenhalt, Unterstützung, Motivation und gemeinsame Unternehmungen.
Meinen lieben Eltern und Großeltern mit Geschwistern, sowie meinem lieben Schwesterherz und Freund mit Familie danke ich aus ganzem Herzen für eure jahrelange Unterstützung, Bestätigungen, Aufmunterungen, Motivation, Geduld, Verständnis und den familiären Rückhalt. Ganz herzlichen Dank.
Juliane Schott
i
Table of Contents
page
List of abbreviations, symbols and units iii ─ v
List of Figures vi ─ ix
List of Tables x ─ xi
Summary xii ─ xiii
Zusammenfassung xiv ─ xvi
1 Motivation and Aims 1 ─ 4
2 Basics 5 ─ 28
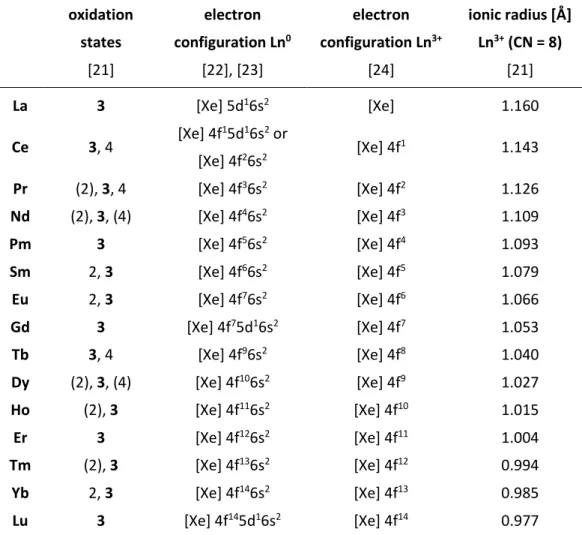
2.1 Trivalent actinides and lanthanides 5 ─ 9
2.2 Complexation reaction of metals with ligands in aqueous solution 9 ─ 13
2.3 Literature overview 13 ─ 26
2.3.1 B(OH)3-(poly)borate equilibria 13 ─ 20
2.3.2 Organoborates 20 ─ 21
2.3.3 Metal-borate complexes 22 ─ 24
2.3.4 Borate solids 24 ─ 26
2.4 Working hypothesis and approaches 26 ─ 28
3 Methods 29 ─ 50
3.1 Luminescence spectroscopy 29 ─ 39
3.1.1 Time-resolved laser-induced fluorescence spectroscopy (TRLFS) 31 ─ 33
3.1.2 Luminescence properties of Eu(III) 33 ─ 39
3.2 NMR spectroscopy 39 ─ 47
3.2.1 Basic principles of NMR spectroscopy 39 ─ 42
3.2.2 11B NMR spectroscopy 42 ─ 47
3.3 Miscellaneous methods and analytics 47 ─ 50
ii
4 Results and Discussion 51 ─ 96
4.1 The system Eu(III)-B(OH)3-polyborates 51 ─ 82
4.1.1 11B NMR spectroscopy of B(OH)3-polyborate containing solutions 51 ─ 53
4.1.2 Eu(III)-(poly)borate complexation 54 ─ 62
4.1.3 Description of a Eu(III)-borate solid 62 ─ 82
4.2 The system Eu(III)-B(OH)3-organics 82 ─ 96
4.2.1 Formation of organoborates 83 ─ 86
4.2.2 Eu(III)-organoborate complexation 86 ─ 95
4.2.3 Effect of organics on the Eu(III)-borate solid formation 95 ─ 96
5 Conclusion and Outlook 97 ─ 98
6 Experimental details 99 ─ 105
Appendix 106 ─ 117
References 118 ─ 126
List of Publications 127 ─ 128
iii
List of abbreviations, symbols and units
α alpha particle
An, An(III) actinides, trivalent actinides
BH, B boric acid, monoborate
BL, BLac, BSal organoborate, lactatoborate, salicylatoborate B(3), B(4) threefold/fourfold coordinated boron center [BO3], [BO4−] trigonal planar/tetrahedral boron units BMWi Bundesministerium für Wirtschaft und Energie
(Federal Ministry for Economic Affairs and Energy)
CN coordination number
δ in-plane bending
DFT density functional theory
DLS dynamic light scattering
e Euler´s number
ED electric dipole
EDTA ethylenediaminetetraacetic acid
EDX energy dispersive X-ray analysis
efg electric field gradient
e.g. for example
Eq. equation
et al. and others
etc. et cetera
EuL, EuL2, EuBL 1:1/1:2 Eu(III)-ligand complex, Eu(III)-organoborate complex EuLac, EuLac2, EuBLac 1:1/1:2 Eu(III)-lactate complex, Eu(III)-lactatoborate complex EuSal, EuSal2, EuBSal 1:1/1:2 Eu(III)-salicylate complex, Eu(III)-salicylatoborate complex
FID free induction decay
Fig. figure
FT Fourier transform
γ out-of-plane bending
HLW high-level radioactive waste
HSAB hard and soft acids and bases (concept of Pearson)
HZDR Helmholtz-Zentrum Dresden-Rossendorf
iCCD intensified charge-coupled device
ICP-MS Inductively Coupled Plasma Mass Spectrometry
i.e. that is
IR infrared
IUPAC International Union of Pure and Applied Chemistry LacH, Lac lactic acid, lactate
laser light amplification by stimulated emission of radiation
lg decadic logarithm
LH, L protonated ligand, deprotonated ligand Ln, Ln(III) lanthanides, trivalent lanthanides
M metal ion
MAS magic angle spinning
MD magnetic dipole
ML metal-ligand complex
n neutron
Nagra Nationale Genossenschaft für die Lagerung radioaktiver Abfälle (National Cooperative for the Disposal of Radioactive Waste) Nd:YAG neodymium-doped yttrium aluminium garnet
iv
NEA Nuclear Energy Agency
NMR nuclear magnetic resonance (spectroscopy)
OECD Organisation for Economic Co-operation and Development
OPO optical parametric oscillator
PARAFAC parallel factor analysis
PSI Paul Scherrer Institut
S singlet state
SalH, Sal salicylic acid, salicylate
SEM Scanning Electron Microscopy
SIT Specific Ion Interaction Theory
T triplet state
TDB thermodynamic data base
THEREDA Thermodynamic Reference Database
TRLFS time-resolved laser-induced fluorescence spectroscopy
UV/Vis ultraviolet/visible
ν stretching vibration
viz. that is
WIPP Waste Isolation Pilot Plant
XRD X-ray diffraction
[…] reference
ai activity of species i
B, B0 magnetic field, external magnetic field
[B]total total concentration of boron
β, βm, β0 cumulative complexation constant in molar scale, in molal scale, extrapolated to zero ionic strength
c concentration in molar scale; speed of light
d thickness of the sample
D Debye-Hückel term
∆ difference
δ chemical shift
E energy
ε decadic molar extinction coefficient; interaction coefficient
Eh redox potential
[Eu(III)]total total concentration of Eu(III)
F1/F2 intensity ratio of 5D0→7F1 and 5D0→7F2 transition
γ activity coefficient; gyromagnetic ratio
h Planck´s constant
I, Im ionic strength in molar scale, in molal scale; nuclear spin quantum number I0, IA, IL intensity of initial light, absorbed light, luminescence light
J total angular momentum
k decay rate
K, K0 formation constant in molar scale, extrapolated to zero ionic strength;
stepwise complexation constant
Ka, Ka0, pKa acid dissociation constant in molar scale, extrapolated to zero ionic strength, negative decadic logarithm of Ka
Kw, pKw ion product of water, negative decadic logarithm of Kw
L, l total orbital angular momentum, orbital angular momentum
λ, λex, λem wavelength, excitation wavelength, emission wavelength [lactate]total total concentration of lactate (Lac + LacH)
m concentration in molal scale;
magnetic quantum number
v
M multiplicity
µ magnetic moment
nH2O amount of water molecules in the first hydration shell [organics]total total concentration of organics
P nuclear spin
p, pCO2 pressure, partial pressure of CO2
pH, pHc (corrected) negative decadic logarithm of the hydrogen ion activity or concentration
Q quantum yield
ρ conversion factor
S, s total spin, electron spin
σ standard deviation
[salicylate]total total concentration of salicylate (Sal + SalH)
t time
T temperature
T1/2 half-life
τ luminescence lifetime
ν frequency, wavenumber; stoichiometric coefficient
x fraction
z charge
[X] equilibrium concentration of substance X
Å Ångström (1 Å = 10−10 m)
a.u. arbitrary unit
cm centimeter
d day
g gram
K, °C Kelvin, degree Celsius
kg kilogram
L liter
m mol/kg
m³ cubic meter
M mol/L
MHz megahertz
mol mol
MPa megapascal
ms millisecond
µs microsecond
nm nanometer
ppm parts per million
rad radian
s second
T tesla
% percent
vi
List of Figures
Fig. 1: Molecular structure of the main and clearly identified polyborate species in solution.
Fig. 2: B(OH)3-polyborate speciation relative to [B]total for different [B]total as a function of pH, I = 0.1 M (NaClO4). Calculation with converted values of Ingri´s formation constants [46], [48] for different (poly)borates (Table A-1, see appendix). Calculated B(OH)3-polyborate formation as effective species concentration is shown in Fig. A-1 (see appendix).
Fig. 3: Distribution of the polyborates B3O3(OH)4− (left) and B5O6(OH)4− (right) as a function of [B]total and pH, I = 0.1 M (NaClO4).
Fig. 4: General structures of organoborates resulting from reaction of (a) boric acid with hydroxycarboxylates and (b) monoborate with polyols.
Fig. 5: Reaction mechanism for the formation of the (a) mono-cyclic and (b) bi-cyclic organoborate (hydroxycarboxylate based).
Fig. 6: Linear relationship (according to Bousher [83]) between complexation constants of metal-borate complexes (converted values according to Eq. 26) and first metal hydrolysis constants (converted values according to Eq. 24), Table 6. The calculated data points for Eu(III) from this relationship are plotted and respective values for lg β*Eu,B/lg β0Eu,B are calculated.
Fig. 7: M(III)-borate complex, M = trivalent actinides/lanthanides.
Fig. 8: Jabłoński diagram, according to Otto [114].
Fig. 9: Set-up of the laser system for luminescence spectroscopy.
Fig. 10: Time-resolved Eu(III) luminescence spectra (left), decay curve of the Eu(III) luminescence (right).
Fig. 11: Energy level diagram of Eu(III). The coupling of the lowest excited state of Eu(III) with an overtone frequency of water leads to luminescence quenching. Figure according to Horrocks et al. [116]
with data of Carnall et al. [115].
Fig. 12: (a) Luminescence spectrum of Eu(III) aquo ion, (b) Luminescence spectrum of Eu(III) in presence of a ligand (here: [salicylate]total = 0.01 M, pH 5).
Fig. 13: 11B ground state splitting in an external magnetic field.
Fig. 14: Figure copied from Müller et al. [128]; 11B MAS NMR spectrum of Tl[B5O6(OH)4]·2H2O (128.3 MHz), (a) experimentally obtained spectrum, (b) calculated spectrum, (c) single components of the spectrum, (d) spectrum calculated for 160.4 MHz.
Fig. 15: 11B NMR spectra (normalized) of solutions containing variable amounts of [B]total (0.2 m to 0.7 m, step size 0.1 m) at (a) pHc 5 and (b) pHc 6; in each case Im = 0.1 m (NaClO4). The insets show expansion of the polyborate region. 11B NMR spectrum in orange shows a six months aged solution containing [B]total = 0.7 m at pHc 6, Im = 0.1 m (NaClO4).
Fig. 16: Europium luminescence spectra at (a) pHc 5 and (b) pHc 6 as a function of [B]total (step size 0.1 m), 3·10-5 m Eu(III), Im = 0.1 m (NaClO4).
Fig. 17: F1/F2 as a function of pHc, pH titration of solutions containing 3·10-5 m Eu(III) and [B]total = constant, Im = 0.1 m (NaClO4).
Fig. 18: Linear relationship (according to Bousher [83], see chapter 2.3.3) between complexation constants of metal-borate complexes (converted value according to Eq. 26) and first metal hydrolysis constants (converted value according to Eq. 24), Table 6. The determined data points for Eu(III) (this work) integrate well into this relationship.
vii
Fig. 19: Eu(III)-(poly)borate speciation as a function of [B]total and pH (carbonate-free system), [Eu(III)]total
= 3·10-5 m, Im = 0.1 m (NaClO4). Speciation calculated with converted complexation constants summarized in Table A-1 (see appendix). Application of lg βEuB = 2.0 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Eu(III)- (poly)borate speciation calculated with lg βEuB = 2.6: Fig. A-2 (see appendix).
Fig. 20: F1/F2 ratio as a function of [B]total and ionic strength, left: NaClO4 medium, right: NaCl medium.
Fig. 21: (a) SIT plot of data points from the Eu(III)-(poly)borate-NaClO4 system (error bars: 2σ from estimation), (b) SIT curve (calculated with lg β01 = 3.14 and ∆ε = -0.09 via Eq. 12) for the Eu(III)- (poly)borate-NaClO4 system.
Fig. 22: left: Development of Eu(III) luminescence spectra and lifetimes with observation time at (a) pHc = 5 and (b) pHc = 6; right: Eu(III) luminescence spectra of unfiltered and filtered solutions at (c) pHc
= 5 and (d) pHc = 6, filtration after 71 days; solution: [B]total = 0.6 m, [Eu(III)]total = 3·10−5 m, Im = 0.1 m (NaClO4).
Fig. 23: Possible Eu(III) environment in the Eu(III)-borate solid; R = H, other threefold coordinated boron centers, condensed borate structures.
Fig. 24: (a) Formation progress of the Eu(III)-borate solid species for a solution containing [Eu(III)]total = 3·10-5 m, [B]total = 0.7 m, Im = 0.1 m (NaClO4) at pHc 6, (b) Europium luminescence lifetime observed with observation time.
Fig. 25: Eu(III) luminescence decay curves during the formation progress of the Eu(III)-borate solid at pHc
= 6; [Eu]total = 3·10-5 m, [B]total = 0.7 m, Im = 0.1 m (NaClO4).
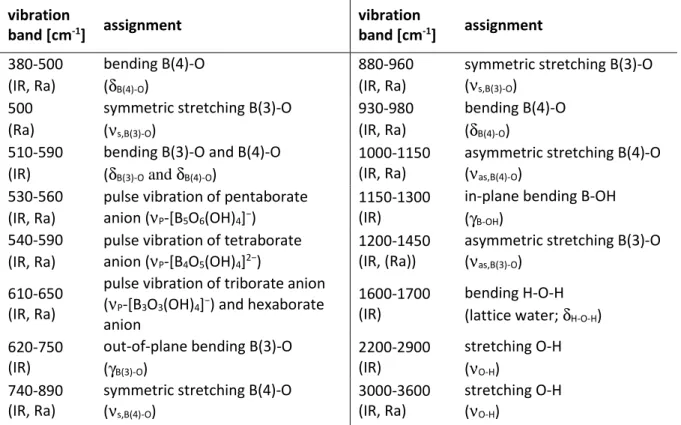
Fig. 26: IR spectrum of the isolated Eu(III)-borate solid and for comparison of boric acid in the range of (a) 4000-380 cm-1 and (b) 800-380 cm-1.
Fig. 27: IR spectrum of the isolated Eu(III)-borate solid precipitated at different ionic strengths and media (NaCl/NaClO4), above: comparison of ionic strength, below: comparison of medium.
Fig. 28: Solid-state 11B NMR spectrum (256.8 MHz) of the La(III)-borate solid as representative for the Eu(III)-borate solid.
Fig. 29: Solid-state Eu(III) luminescence spectra of the Eu(III)-borate solid precipitated at different ionic strengths and media (left: NaClO4, right: NaCl). Excitation: λex = 394 nm, room temperature (T = 22 °C).
Fig. 30: Excitation spectra at low temperature (T < 5 K) of the Eu(III)-borate solid precipitated at different ionic strengths and media (NaClO4/NaCl).
Fig. 31: Comparison of the Eu(III) luminescence spectra at low temperature (T < 5 K) of the Eu(III)-borate solid excited at different λex; Eu(III)-borate solid precipitated at different ionic strengths and media (NaCl/NaClO4).
Fig. 32: Luminescence spectra of single Eu(III) species determined with PARAFAC from luminescence data.
Fig. 33: Content of different Eu(III) solid species in the Eu(III)-borate solid precipitated at different ionic strengths.
Fig. 34: Eu(III) luminescence spectra of unfiltered and filtered solutions (left: spectrum with detected intensity, right: spectrum with normalized intensity); filtration after around 1 year and 3 months;
solution: [B]total = 0.5 m, [Eu(III)]total = 3·10−5 m, pHc = 6, Im = 0.1 m (NaClO4).
viii
Fig. 35: SEM images of Eu(III)-borate particles on a 50 nm-membrane filter surface (black spots = 50 nm pores).
Fig. 36: (a) salicylatoborate, (b) lactatoborate.
Fig. 37: 11B NMR spectra of aqueous solutions (pH 5, I = 0.1 M (NaClO4)) with (a) [B]total = 0.2 M and [lactate]total = 0.005 M lactate, (b) [B]total = 0.2 M and [salicylate]total = 0.005 M; scaling factors of the organoborate signals are given.
Fig. 38: Speciation of different organic-boron systems for solutions with [organics]total = 0.005 M and [B]total = 0.2 M, I = 0.1 M (NaClO4), T = 22 °C (own data for pKa and KBL taken from Table 25 and Table 26). left: salicylate-B(OH)3 system, right: lactate-B(OH)3 system. Speciations relative to [B]total: Fig. A-9 (see appendix).
Fig. 39: Influence of boric acid on the Eu(III)-salicylate system. Eu(III) luminescence spectra of solutions at around pH 4.4 and [Eu(III)]total = 3·10−5 M, I = 0.1 M (NaClO4) with (I) [salicylate]total = 0.01 M, (II) [salicylate]total = 0.01 M and [B]total = 0.2 M, (III) pH titration of a solution containing [Eu(III)]total = 3·10−5 M, [salicylate]total = 0.01 M and [B]total = 0.2 M from around pH 4.4 down to around pH 2.
Fig. 40: 11B chemical shift of salicylatoborate as a function of [Eu(III)]total; solutions: [salicylate]total = 0.005 M, [B]total = 0.2 M, [Eu(III)]total = variable, pH 5, 11B chemical shift corrected according to [108].
Fig. 41: Influence of boric acid on the Eu(III)-lactate system. Eu(III) luminescence spectra of solutions at around pH 4.4 and [Eu(III)]total = 3·10−5 M, I = 0.1 M (NaClO4) with (I) [lactate]total = 0.002 M, (II) [lactate]total = 0.002 M and [B]total = 0.4 M, (III) pH titration of a solution containing [Eu(III)]total = 3·10−5 M, [lactate]total = 0.002 M and [B]total = 0.4 M from around pH 4.4 down to around pH 2.
Fig. 42: 11B chemical shift of lactatoborate as a function of [Eu(III)]total; solutions: [lactate]total = 0.005 M, [B]total = 0.2 M, [Eu(III)]total = variable, pH 5, 11B chemical shift corrected according to [108].
Fig. 43: Speciation for the Eu(III)-salicylate-B(OH)3 system as a function of [salicylate]total and pH (carbonate-free system), [Eu(III)]total = 3·10-5 M, I = 0.1 M (NaClO4). Speciation calculated with converted complexation constants summarized in Table A-1 (see appendix). Application of lg βEuBSal = 2.0, and lg βEuB = 2.0 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Speciation for the Eu(III)-salicylate-B(OH)3 system calculated with lg βEuBSal = 2.7 and lg βEuB = 2.6: Fig. A-13 (see appendix).
Fig. 44: Speciation for the Eu(III)-lactate-B(OH)3 system as a function of [lactate]total and pH (carbonate- free system), [Eu(III)]total = 3·10-5 M, I = 0.1 M (NaClO4). Speciation calculated with converted complexation constants summarized in Table A-1 (see appendix). Application of lg βEuBLac = 2.0, and lg βEuB = 2.0 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Speciation for the Eu(III)-lactate-B(OH)3 system calculated with lg βEuBLac
= 2.7 and lg βEuB = 2.6: Fig. A-14 (see appendix).
Fig. 45: Eu(III)-borate solid formation progress as a function of [organics]total; solution: [Eu(III)]total = 3·10-5 M, [B]total = 0.65 M, [oranganics]total = variable, pH = 6, I = 0.1 M (NaClO4).
Fig. A-1: B(OH)3-polyborate formation as effective species concentration for different [B]total as a function of pH, I = 0.1 M (NaClO4). Calculation with converted values of Ingri´s formation constants [46], [48] for different (poly)borates (Table A-1, see appendix).
Fig. A-2: Eu(III)-(poly)borate speciation as a function of [B]total and pH (carbonate-free system), [Eu(III)]total
= 3·10-5 m, Im = 0.1 m. Speciation calculated with converted complexation constants summarized
ix
in Table A-1 (see appendix). Application of lg βEuB = 2.6 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Eu(III)-(poly)borate speciation calculated with lg βEuB = 2.0: see Fig. 19.
Fig. A-3: Powder X-ray diffraction pattern of the Eu(III)-borate solid (black graph); grey vertical lines: main diffraction peaks of the sodium pentaborate phase (Na2[B5O8(OH)]·H2O) described by Menchetti et al. [146].
Fig. A-4: Comparison of the IR spectrum of the Eu(III)-borate and La(III)-borate solid precipitated at I = 0.1 m (NaClO4).
Fig. A-5: Proposed structures of the borate ligand in the Eu(III)-borate solid deduced from solid-state
11B NMR spectroscopy.
Fig. A-6: Europium luminescence spectra obtained at low temperature (left: λex = 578.5 nm, right: λex = 579.45 nm) of the Eu(III)-borate solid precipitated at different ionic strengths (0.1 m/1 m/3 m NaCl/NaClO4).
Fig. A-7: Luminescence spectra of single Eu(III) species determined with PARAFAC from luminescence data, 5D0 →7F3 transition is shown.
Fig. A-8: Particle size distribution of colloid-like particles (in the 1.2 µm filtrate) of Eu(III) and polyborate containing solutions.
Fig. A-9: Speciation of different organic-boron systems for solutions with [organics]total = 0.005 M and [B]total = 0.2 M, I = 0.1 M, T = 22 °C (own data for pKa and KBL taken from Table 25 and Table 26, Table A-1, see appendix). (a) salicylate-B(OH)3 system, (b) lactate-B(OH)3 system.
Fig. A-10: (a) Eu(III)-salicylate speciation at pH 5 and I = 0.1 M (NaClO4); [Eu(III)]total = 3·10−5 M Eu(III), [salicylate]total = 0.0029 M (if [B]total = 0.2 M, [salicylate]total = 0.01 M → [salicylate]free =
0.0029 M); lg βEuSal = 2.10 (this work), lg βEuSal2 = 3.84 [34]. (b) Eu(III)-lactate speciation at pH 5;
[Eu(III)]total = 3·10−5 M Eu(III), [lactate]total = 0.0056 M (if [B]total = 0.2 M, [lactate]total = 0.01 M → [lactate]free = 0.0056 M); lg βEuLac = 2.51 [33], lg βEuLac2 = 4.45 [33].
Fig. A-11: Europium(III) speciation obtained from PARAFAC. Solutions: [Eu(III)]total = 3·10−5 M , [salicylate]total = 0.01 M and varying [B]total , pH 5, I = 0.1 M (NaClO4).
Fig. A-12: Europium(III) speciation obtained from PARAFAC. Solutions: [Eu(III)]total = 3·10−5 M, [lactate]total = 0.01 M and varying [B]total, pH 5, I = 0.1 M (NaClO4).
Fig. A-13: Speciation for the Eu(III)-salicylate-B(OH)3 system as a function of [salicylate]total and pH (carbonate-free system), [Eu(III)]total = 3·10-5 m, I = 0.1 m (NaClO4). Speciation calculated with converted complexation constants summarized in Table A-1 (see appendix). Application of lg βEuBSal = 2.7, and lg βEuB = 2.6 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Speciation for the Eu(III)-salicylate-B(OH)3 system calculated with lg βEuBSal = 2.0 and lg βEuB = 2.0: see Fig. 43.
Fig. A-14: Speciation for the Eu(III)-lactate-B(OH)3 system as a function of [lactate]total and pH (carbonate- free system), [Eu(III)]total = 3·10-5 m, I = 0.1 m (NaClO4). Speciation calculated with converted complexation constants summarized in Table A-1 (see appendix). Application of lg βEuBLac = 2.7, and lg βEuB = 2.6 (primarily valid for Eu(III) complexes with borate ligands having one binding site) for all the Eu(III)-(poly)borate complexes, although there are Eu(III) complexes with borate ligands having two binding sites. Speciation for the Eu(III)-lactate-B(OH)3 system calculated with lg βEuBLac
= 2.0 and lg βEuB = 2.0: see Fig. 44.
x
List of Tables
Table 1: Oxidation states and electron configurations of lanthanides; electron configurations and ionic radii of trivalent lanthanides.
Table 2: Oxidation states and electron configurations of actinides; electron configurations and ionic radii of trivalent actinides.
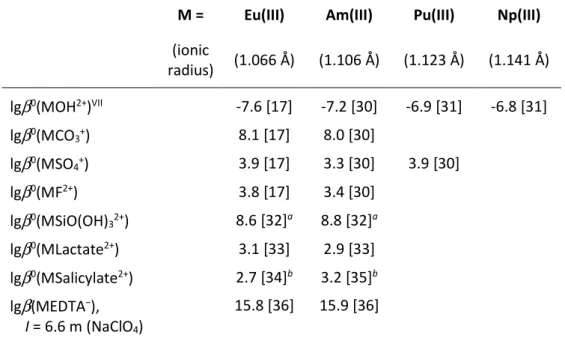
Table 3: Complexation constants of trivalent europium and trivalent actinides with inorganic and organic ligands.
Table 4: Overview about theories describing ion interactions in (concentrated) solutions and determination of γi.
Table 5: Polyborate formation constants according to Eq. 15 and Eq. 17 to 20. Formation constants in bold are applied in this work.
Table 6: Metal hydrolysis constants βM,OH according to Eq. 22, and metal-borate complexation constants βM,B according to Eq. 21 as well as converted values (pβ*M,OH according to Eq. 23/24 and lg β*M,B according to Eq. 25/26).
Table 7: Vibration frequencies of borate compounds and assignment according to Janda et al. [90] and Li Jun et al. [91].
Table 8: Some Eu(III) energy levels and corresponding energies. Data from Carnall et al. [115].
Table 9: Characterization of Eu(III) luminescence transitions, according to Bünzli et al. [117] and Binnemans [118].
Table 10: Deduction of Eu(III) symmetry from 5D0→7FJ transition splitting pattern, according to Bünzli et al.
[117] and Binnemans [118].
Table 11: Spin, gyromagnetic ratio and natural abundance of several nuclei, data from [125].
Table 12: Fractions of B(OH)3 and polyborate species in solution at pHc = 6 as a function of [B]total and ionic strength.
Table 13: Europium luminescence lifetime τ (overall Eu(III) species) at pHc 6 as a function of [B]total, [Eu(III)]total = 3·10-5 m, Im = variable, electrolyte: NaClO4.
Table 14: Average lg β1,m (I = 0.1 m (NaClO4/NaCl)) and corresponding lg β01 (extrapolation to infinite dilution according to Davies approach [37]) of the Eu(III)-borate complex, EuB(OR)42+ (borate ligand with one binding site) according to Eq. 45. T = 22 °C. Uncertainty: 2σ.
Table 15: lg β1,m values of the Eu(III)-borate complex, EuB(OR)42+ (borate ligand with one binding site), as a function of ionic strength (NaClO4), [Eu(III)]total = 3·10-5 m, [B]total = variable, pHc = 6, T = 22 °C;
averaged deviation for lg β1,m (estimated): 0.2 (2σ).
Table 16: Summarized values for lg β01, ∆ε and ε(EuB(OR)42+, ClO4−) for the Eu(III)-(poly)borate-NaClO4
system.
Deviation: 2σ (from linear fit of the data, Fig. 21a).
Table 17: Time period of the first indication of the Eu(III)-borate solid formation as a function of [B]total and ionic strength, medium: NaCl; grey: outlier. Values in days (d).
Table 18: Time period of the first indication of the Eu(III)-borate solid formation as a function of [B]total and ionic strength, medium: NaClO4; grey: outlier. Values in days (d).
Table 19: Grade of completeness of the conversion of dissolved Eu(III) ([Eu(III)]total = 3·10−5 m) into the Eu(III)-borate solid as a function of [B]total and ionic strength, medium: NaCl; analysis of filtrates with TRLFS after sample filtration with membrane filters of 0.2 µm pore size; sample treatment after around 6 months.
xi
Table 20: Grade of completeness of the conversion of dissolved Eu(III) ([Eu(III)]total = 3·10−5 m) into the Eu(III)-borate solid as a function of [B]total and ionic strength, medium: NaClO4; analysis of filtrates with TRLFS after sample filtration with membrane filters of 0.2 µm pore size; sample treatment after around 1 year and 3 months.
Table 21: Observed vibration frequencies in the IR spectra of boric acid and the isolated Eu(III)-borate solid.
Table 22: Luminescence lifetimes of the Eu(III)-borate solid for excitations at different λex.
Table 23: Identification of samples with colloid-like particles, medium: NaCl. Sample treatment after around 6 months.
Table 24: Identification of samples with colloid-like particles, medium: NaClO4. Sample treatment after around 1 year and 3 months.
Table 25: pKa values of organic acids and boric acid (according to Eq. 46 and Eq. 47), determined within this work (T = 22 °C, in bold) and from literature (T = 25 °C); deviation: 2σ.
Table 26: Average formation constants KBL (according to Eq. 48) of salicylatoborate and lactatoborate determined within this work (values in bold), pH = 5, I = 0.1 M (NaClO4), T = 22 °C, deviation: 2σ. Table 27: Eu(III) complexation constants βEuL and βEuL2 (according to Eq. 49 and Eq. 50), I = 0.1 M (NaClO4),
T = 22-25 °C, deviation: 2σ.
Table 28: Eu(III)-salicylatoborate complexation constants βEuBSal (according to Eq. 51); I = 0.1 M, T = 22 °C;
deviation: 2σ.
Table 29: Eu(III)-lactatoborate complexation constants βEuBLac (according to Eq. 51); I = 0.1 M, T = 22 °C;
deviation: 2σ.
Table A-1: Applied complexation constants for speciation calculations.
Table A-2: Influence of the organoborate ring structure on the organoborate 11B chemical shift.
Table A-3: lg β1 values (according to Eq. 45) of the Eu(III)-borate complex, EuB(OR)42+ (borate ligand with one binding site), for different data sets; [Eu(III)]total = 3·10-5 m, I = 0.1 m (NaClO4/NaCl), T = 22 °C.
Table A-4: Formation constants KBL (according to Eq. 48) of salicylatoborate and lactatoborate for different composed solutions determined from 11B NMR data, pH = 5, I = 0.1 M, T = 22 °C.
Table A-5: Complexation constants βEuBSal according to Eq. 51 for different TRLFS data sets; [Eu(III)]total = 3·10-5 M, I = 0.1 M, T = 22 °C; error of fit: 0.02 (average); deviation of averaged value: 2σ. Table A-6: xfree and xcomplex deduced from 11B NMR spectroscopic data [106], T = 22 °C, pH 5; (a)
[salicylate]total = 0.005 M, [B]total = 0.2 M, (b) [salicylate]total = 0.01 M, [B]total = 0.2 M, (c) [lactate]total = 0.005 M, [B]total = 0.2 M; xfree: fraction of free organoborate, xcomplex: fraction of Eu(III) bound organoborate.
xii
Summary
The aim of this work is the characterization of the Eu(III)-borate system by the identification of possible interactions (Eu(III) complexation by borate ligands, Eu(III) precipitation, etc.). Borate compounds will be a part of the inventory of a future nuclear waste repository, e.g., glass coquilles for high-level radioactive waste and cementitious waste packages. Moreover, borate compounds naturally occur in rock salt, a potential host rock formation for nuclear waste repositories, e.g., as minerals (borax, etc.), and in respective brines. Hence, the interactions of borate compounds with components of nuclear waste, i.e., radionuclides (e.g., actinides like Am and Pu), is of general interest in the identification and characterization of mobilization pathways of radionuclides in the context of the safety and risk assessment for a future nuclear waste repository. Until now, the complexation between actinides and borate species is investigated insufficiently. Eu(III) is available from non-radioactive compounds and is used as a chemical analog for trivalent actinides, e.g., Am(III) and Pu(III). Consequently, the handling with Eu(III) enables easier access to and extensive studies in the unknown An(III)/Ln(III)-borate complexation system.
Two different approaches to study the Eu(III)-borate complexation system were drafted to determine the order of magnitude for the Eu(III)-borate complexation constant. For that purpose the Eu(III) complexation with various borate ligands holding a „B(OR)4−“ unit was investigated. For the first approach inorganic borate compounds, i.e., polyborates, and for the second approach organoborates (salicylatoborate and lactatoborate) were used. A similar complexation behavior of borate ligands with one „B(OR)4−“ unit concerning Eu(III) was hypothesized and experimentally confirmed. In the complexation studies different spectroscopic techniques were applied, for instance time-resolved laser-induced fluorescence spectroscopy (TRLFS) and NMR spectroscopy. As a result of these complexation studies the complexation constant for a 1:1 Eu(III)-borate complex (EuB(OR)42+) is set in the range lg β0 = 2.6 – 3.3. Hence, the Eu(III)-borate complexation is weak. SIT (Specific Ion Interaction Theory) parameters were determined in this work from the ionic strength dependency of the Eu(III)- (poly)borate complexation constant in NaClO4 medium (lg β01 = 3.14 ± 0.17, ∆ε = (-0.09 ± 0.10) kg/mol, ε(EuB(OR)42+, ClO4−) = 0.33 kg/mol).
Prior the Eu(III)-borate complexation studies the borate speciation in solution was studied with
11B NMR spectroscopy. For the first approach the formation of polyborate species was studied. At pHc 6 triborate and pentaborate species were detected. Their formed amount agrees well with calculated amounts from polyborate formation constants found in literature. For the second approach the formation of organoborates (salicylatoborate and lactatoborate) was studied and their formation constants were determined (lg K0BSal = 1.10 ± 0.07, lg K0BLac = 0.57 ± 0.11).
xiii
In the Eu(III)-polyborate system at pHc 6 a Eu(III) precipitation was observed by means of TRLFS. IR and solid-state 11B NMR spectroscopy confirmed the formation of a borate containing Eu(III) solid phase.
At low Eu(III) concentrations (3·10−5 m) this Eu(III)-borate precipitate forms within days to weeks depending on [B]total and the medium (background electrolyte, ionic strength). The higher [B]total and the Eu(III) concentration the faster is the solid formation. The Eu(III)-borate solid phase is long-term stable at pHc 6, but dissolves increasingly with decreasing pH. An effect of the presence of organics (salicylate, lactate) on the precipitation kinetics was not detected up to [organics]total = 2·10−3 m. For [lactate]total = 2·10−2 m a delay of the Eu(III)-borate precipitation progress and for [salicylate]total = 2·10−2 m an incomplete conversion of the dissolved Eu(III) into the solid were observed.
The isolated Eu(III)-borate solid is amorphous. Hence, structural information from X-ray diffraction is not obtainable. The analysis of the solid-state 11B NMR spectrum of a La(III)-borate solid (structural similarity to the Eu(III)-borate solid confirmed with IR spectroscopy) reveals a ratio of the fourfold coordinated to the threefold coordinated boron environments of 3:1 in the borate ligand.
Unfortunately, from this information no concrete structure of the borate ligand around La(III) is deducible. However, the high amount of fourfold coordinated boron environments suggests a high(er) grade of condensation of the borate structure.
Three europium species in the solid were identified by solid-state site-selective TRLFS investigations of the Eu(III)-borate solid. Luminescence spectrum and luminescence lifetime of two species are quite similar. They are assigned to the Eu(III)-borate species. Both species occur in equal parts if the Eu(III)- borate solid is precipitated at low ionic strength (0.1 m NaClO4/NaCl), whereas high(er) ionic strengths (1 m/3 m NaClO4/NaCl) favors the formation of only one of these species. The IR spectra of the Eu(III)- borate solids precipitated at different ionic strengths and electrolytes are almost identical. Hence, a high structural similarity of these two species can be assumed. Differences are possible in the content of structural water, grade of condensation of the borate ligand or long-range order in the solid. The luminescence spectrum and luminescence lifetime of the third species differ significantly from that of the other species. Possibly, the third species is a primary stage of the completely formed Eu(III)-borate solid.
At Im = 0.1 m (NaCl/NaClO4) colloid-like Eu(III)-borate particles (particle size distribution: 100-500 nm) were detected.
Summarizing the results: Within this work different interactions in the Eu(III)-borate system (complexation, solid formation, colloid formation) were identified and described qualitatively and quantitatively.
xiv
Zusammenfassung
Ziel dieser Arbeit ist die Beschreibung des Eu(III)-Borat Systems durch die Identifizierung möglicher Wechselwirkungen (Komplexierung von Eu(III) mit Boratliganden, Eu(III)-Fällung, etc.).
Boratverbindungen werden Bestandteil des Inventars eines zukünftigen nuklearen Endlagers (Glaskokillen für hochradioaktive Abfälle, Abfallgebinde in Zement, etc.) sein. Des Weiteren sind Boratverbindungen im Steinsalz ─ einer potentiellen Wirtsgesteinsformation für Endlager radioaktiver Abfälle ─ in Form von Mineralen wie Borax und Salzlaugen natürlich vorhanden. Deshalb sind im Zuge der Risiko- und Sicherheitsanalyse von zukünftigen Endlagern für radioaktive Abfälle im Zusammenhang mit der Identifizierung von Mobilisierungspfaden von Radionukliden die Wechselwirkungen von Boratverbindungen mit Bestandteilen radioaktiver Abfälle, d.h. Radionukliden wie Actinide (z.B. Am und Pu), von generellem Interesse. Bisher ist die Komplexierung von Actiniden mit Boratspezies jedoch nur unzureichend untersucht worden. Eu(III) ist in Form nicht-radioaktiver Verbindungen verfügbar und wird als ein chemisches Analogon für dreiwertige Actinide, wie Am(III) und Pu(III), genutzt. Somit ermöglicht der Umgang mit Eu(III) einen einfacheren Zugang zu und umfangreiche Untersuchungen in dem unbekannten An(III)/Ln(III)-Borat Komplexierungssystem.
Zwei verschiedene Herangehensweisen für die Untersuchung des Eu(III)-Borat Komplexierungssystem wurden erarbeitet, um die Größenordnung für die Eu(III)-Borat Komplexierungskonstante zu bestimmen. Dazu wurde die Eu(III)-Komplexierung mit verschiedenen Boratliganden, die eine
„B(OR)4−“-Einheit aufweisen, untersucht. Für den ersten Untersuchungsansatz wurden anorganische Boratverbindungen, d.h. Polyborate, und für den zweiten Untersuchungsansatz Organoborate (Salicylatoborat und Lactatoborat) verwendet. Ein ähnliches Komplexierungsverhalten der verschiedenen Boratliganden mit einer „B(OR)4−“-Einheit gegenüber Eu(III) wurde angenommen und konnte experimentell bestätigt werden. In den Komplexierungsstudien kamen verschiedene spektroskopische Methoden, z.B. zeitaufgelöste Laser-induzierte Fluoreszenzspektroskopie (TRLFS) und NMR-Spektroskopie, zum Einsatz. Als Ergebnis der Komplexierungsstudien konnte die Komplexierungskonstante für einen 1:1 Eu(III)-Borat Komplex (EuB(OR)42+) im Bereich lg β0 = 2,6 – 3,3 eingegrenzt werden. Die Eu(III)-Borat Komplexierung ist entsprechend schwach. SIT-Parameter (Specific Ion Interaction Theory) wurden in dieser Arbeit aus der Ionenstärkeabhängigkeit der Eu(III)- (Poly)borat Komplexierungskonstante im NaClO4-Medium bestimmt (lg β0 = 3,14 ± 0,17, ∆ε = (-0,09 ± 0,10) kg/mol, ε(EuB(OR)42+, ClO4−) = 0,33 kg/mol).
Vor den Eu(III)-Borat Komplexierungsstudien wurde die Borat-Speziation in Lösung mittels 11B-NMR- Spektroskopie untersucht. Für den ersten Untersuchungsansatz wurde die Bildung von Polyborat-
xv
Spezies nachvollzogen. Bei pHc 6 wurden Tri- und Pentaboratspezies detektiert. Deren gebildete Menge stimmt gut mit berechneten Werten aus Polyborat-Bildungskonstanten (Literaturwerte) überein. Für den zweiten Untersuchungsansatz wurde die Bildung von Organoboraten (Salicylatoborat und Lactatoborat) untersucht und die jeweiligen Bildungskonstanten bestimmt (lg K0BSal = 1,10 ± 0,07, lg K0BLac = 0,57 ± 0,11).
Im Eu(III)-Polyborat System wurde bei pHc 6 mittels TRLFS eine Europiumfällung beobachtet. IR- und Festkörper-11B NMR-Spektroskopie beweisen die Bildung einer Borat-haltigen Eu(III)-Festphase. Bei niedrigen Eu(III)-Konzentrationen (3·10−5 m) erfolgt die Bildung dieses Eu(III)-Borat Feststoffes je nach [B]total und Medium (Hintergrundelektrolyt, Ionenstärke) innerhalb von Tagen bis Wochen. Je höher [B]total und die Eu(III)-Konzentration sind, desto schneller erfolgt die Feststoffbildung. Die Festphase ist bei pHc 6 langzeitstabil, löst sich jedoch zunehmend mit sinkendem pH. Die Präsenz von Organik (Salicylat, Lactat) hat keine Auswirkung auf den zeitlichen Verlauf der Feststoffbildung bis zu einer totalen Organikkonzentration von 2·10−3 m. Für [Lactat]total = 2·10−2 m wurde eine zeitliche Verzögerung der Eu(III)-Borat Ausfällung und für [Salicylat]total = 2·10−2 m eine unvollständige Umwandlung des gelösten Eu(III) in den Feststoff beobachtet.
Der isolierte Eu(III)-Borat Feststoff ist amorph. Deshalb konnten keine Strukturinformationen aus der Röntgenbeugung erhalten werden. Die Auswertung des Festkörper-11B-NMR-Spektrums eines La(III)- Borat Feststoffes (die strukturelle Übereinstimmung mit dem Eu(III)-Borat Feststoffes wurde IR- spektroskopisch bestätigt) offenbarte ein Verhältnis der vierfach- zu dreifachkoordinierten Borumgebungen von 3:1 im Boratliganden. Daraus lässt sich keine konkrete Struktur des Boratliganden um La(III) ableiten. Jedoch lässt die hohe Menge an vierfachkoordinierten Borumgebungen einen höheren Kondensationsgrad der Boratstruktur vermuten.
Aus den Untersuchungen des Eu(III)-Borat Feststoffes mittels Festkörper-TRLFS über die selektive Anregung einzelner Spezies konnten drei Europium-Spezies im Feststoff identifiziert werden. Zwei Spezies sind sich im Lumineszenzspektrum und Lumineszenzlebensdauer recht ähnlich. Sie werden dem Eu(III)-Borat Feststoff zugeordnet. Beide Spezies liegen zu gleichen Anteilen vor, wenn der Eu(III)- Borat Feststoff bei niedriger Ionenstärke (0.1 m NaClO4/NaCl) gefällt wird. Wird der Eu(III)-Borat Feststoff bei höheren Ionenstärken ausgefällt (1 m/3 m NaClO4/NaCl), dann wird die Bildung von nur noch einer der beiden Spezies begünstigt. Die IR-Spektren der bei verschiedenen Ionenstärken und Elektrolyten gefällten Eu(III)-Borat Feststoffe sind nahezu identisch, sodass von einer hohen strukturellen Ähnlichkeit ausgegangen werden kann. Unterschiede gibt es möglicherweise im Strukturwassergehalt, Kondensierungsgrad des Boratliganden oder in der Fernordnung im Feststoff.
Lumineszenzspektrum und Lumineszenzlebensdauer der dritten Spezies unterscheiden sich deutlich
xvi
von denen der anderen Spezies. Möglicherweise handelt es sich bei der dritten Spezies um eine Vorstufe des vollständig ausgebildeten Eu(III)-Borat Feststoffes.
Bei einer Ionenstärke von 0.1 m (NaCl/NaClO4) wurden Kolloid-artige Eu(III)-Borat Partikel (Partikelgrößenverteilung: 100-500 nm) entdeckt.
Zusammenfassend konnten im Rahmen dieser Arbeit verschiedene Arten der Wechselwirkungen im Eu(III)-Borat System (Komplexierung, Feststoffbildung, Kolloidbildung) identifiziert und qualitativ wie quantitativ beschrieben werden.
![Fig. 6: Linear relationship (according to Bousher [83]) between complexation constants of metal-borate complexes (converted values according to Eq](https://thumb-eu.123doks.com/thumbv2/1library_info/4564834.1599790/43.892.109.679.116.546/relationship-according-bousher-complexation-constants-complexes-converted-according.webp)
![Table 11: Spin, gyromagnetic ratio and natural abundance of several nuclei, data from [125]](https://thumb-eu.123doks.com/thumbv2/1library_info/4564834.1599790/59.892.154.498.221.537/table-spin-gyromagnetic-ratio-natural-abundance-nuclei-data.webp)
![Fig. 15: 11 B NMR spectra (normalized) of solutions containing variable amounts of [B] total (0.2 m to 0.7 m, step size 0.1 m) at (a) pH c 5 and (b) pH c 6; in each case I m = 0.1 m (NaClO 4 )](https://thumb-eu.123doks.com/thumbv2/1library_info/4564834.1599790/71.892.117.777.445.1021/spectra-normalized-solutions-containing-variable-amounts-total-naclo.webp)
![Fig. 17: F 1 /F 2 as a function of pH c , pH titration of solutions containing 3·10 -5 m Eu(III) and [B] total = constant, I m = 0.1 m (NaClO 4 )](https://thumb-eu.123doks.com/thumbv2/1library_info/4564834.1599790/74.892.110.515.816.1116/fig-function-titration-solutions-containing-total-constant-naclo.webp)