Application of the Endoscopic Micro-Inspection Tool QEVO
Volltext
Abbildung
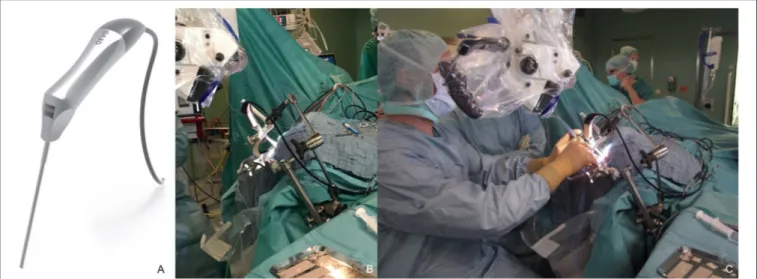
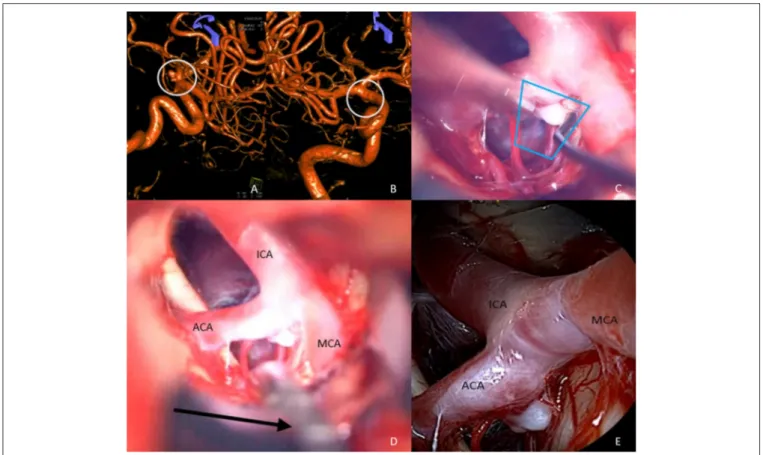
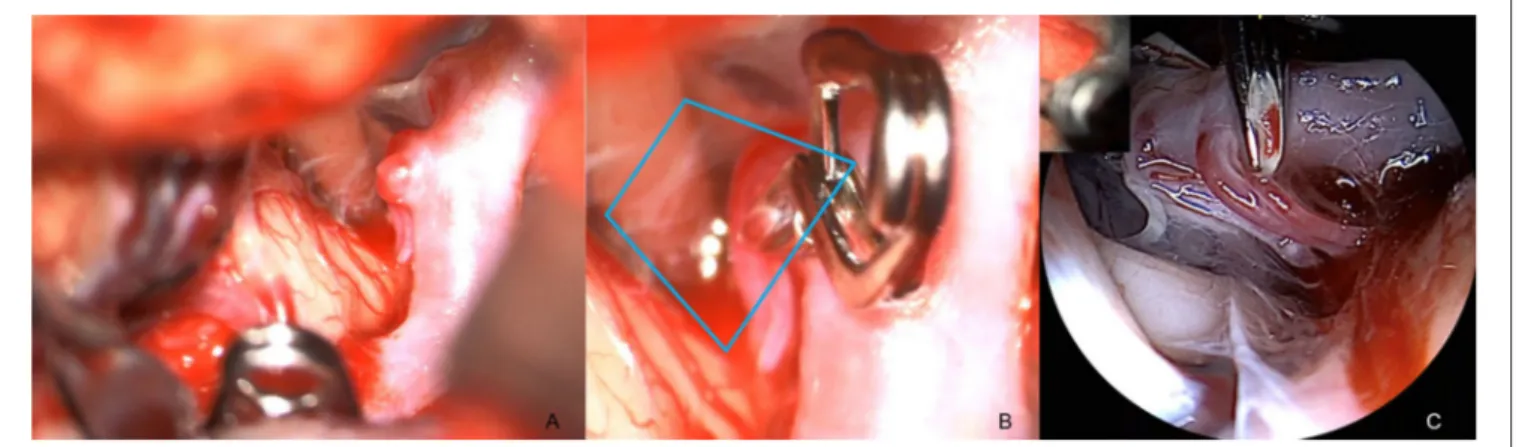
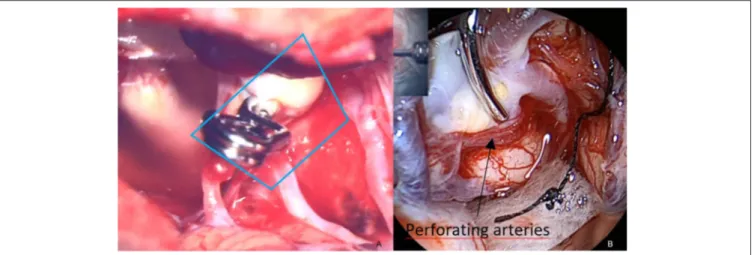
ÄHNLICHE DOKUMENTE
In such cases, artificial neural networks are often used to predict mechanical properties based on mechanical models and experimental data (Chopra et al., 2016).. The prediction
University of Natural Resources and Applied Live Sciences, Institute of Mountain Risk Engineering, Austria (martin.stecher@hotmail.com).. 2
An example of a derivational path within the polyse- mous structure of the verb drop is the following: the primary sense 'let some- thing fall' is derived directly from
– The user defines the final number of control volumes (5 for example) – Grouping based on a connection ratio:. volume of
Attack Surface Vector The attack surface AS of a web application defined according to our construction is a vector consisting of the following components: degree of distribution
The small field of view of the endoscope can be overcome by generating a complete image of the placental surface from the individual fetoscopic images.. In practice, the surgeon
Open period/ Type: Open-Ended Period may result in payment till the day of issue of the medical certificate or be postponed till the end date is established Open period/ End date
The aim of the current paper is to test the applicability of the two-component inspection model to x-ray screening, and to help determine whether an individually