ANOPARTICLES AS C ATALYSTS N E VALUATION OF C OBALTATES AND C OBALT N OBALT -C ATALYZED H YDROGENATIONS : A H OMOGENEOUS VS . H ETEROGENEOUS C
Volltext
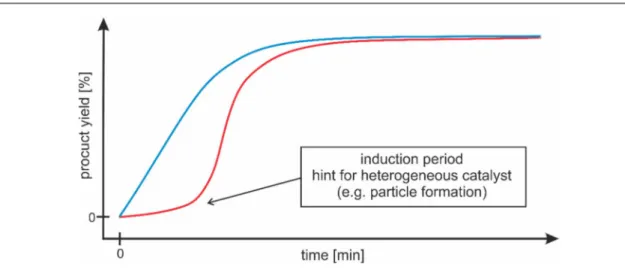
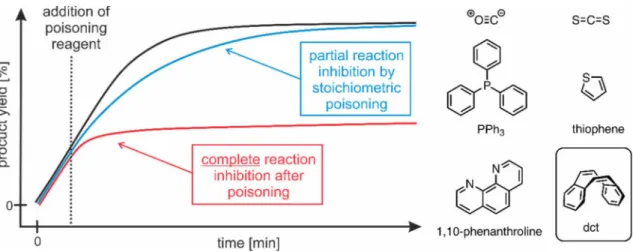
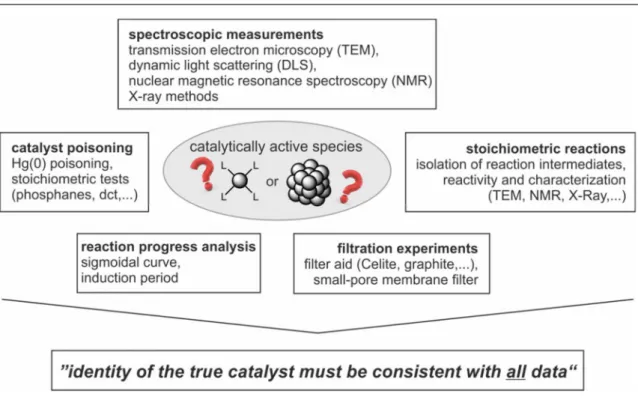
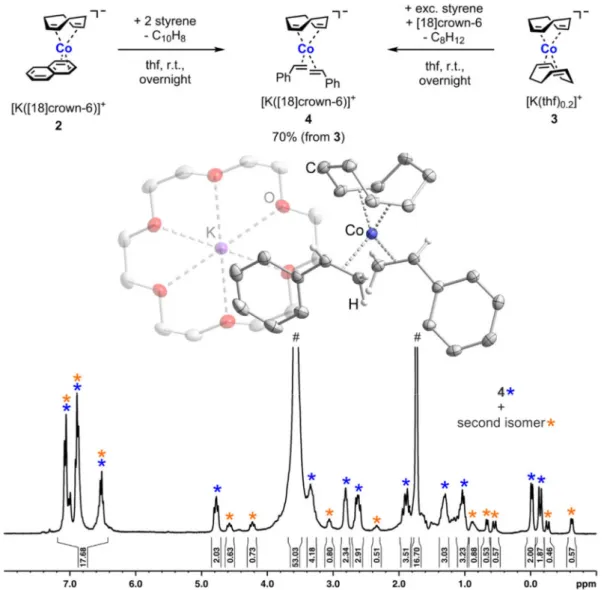
Abbildung

ÄHNLICHE DOKUMENTE
Sondern heut ist der Kamps in j^edes einzelnen Menschen B ru st verlegt : D a ist heut keiner mehr, der nicht christliche Gedanken in sich trüge, auch wenn
Darin hatte er unter anderem Homosexualität als eine „Degenerationsform der Gesellschaft“ bezeichnet und gesagt: „Diese Homolobby, dieses Teuflische kommt immer stärker,
Duck, chicken and beef with Shanghai Paksoi, Bell pepper, broccoli and sweet peas fried in a
Wir haben ja schliesslich im Gegensatz zur Zeit der Spanischen Grippe, unsere hochtechnisierte „Medizin“ und auch ist die Sauberkeit und Hygiene mit der von Anfang des
2 Wenn die Reaktion zu exotherm wird (starkes Sieden des Ethers) muss mit einem Eisbad gekühlt und die Zutropfgeschwindigkeit reduziert werden.. Nach Zugabe des Alde- hyds wird
The most common way to achieve site-selectivity in direct C – H bond activation on arenes is the use of a directing group, which is usually placed in the ortho-position to the C –
che zu bezweifeln hat: so beweiset es, in welcher guten Meinung Pattkul seines Wandels wegen bey andern gestanden haben müsse. Des Churfürsten Johann Georg des
In ein e m zweiten Schritt werden sozio-politische und ökonomische Bedingungen der Entwicklung, Anwendung und Folgebearbeitung unterschiedlicher Nutzungsformen der