Research Collection
Doctoral Thesis
Ecological processes governing the assembly of bacterial root nodule communities in rooibos (Aspalathus linearis)
Author(s):
Ramoneda, Josep Publication Date:
2020
Permanent Link:
https://doi.org/10.3929/ethz-b-000423576
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
DISS. ETH NO. 26544
Ecological processes governing the assembly of bacterial root nodule communities in rooibos (Aspalathus linearis)
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH
(Dr. sc. ETH Zurich)
Presented by
Josep Ramoneda Massagué
MRes Ecology, Evolution and Conservation, Imperial College London
Born on 25.09.1993 Citizen of Catalunya (Spain)
Accepted on the recommendation of Prof. Emmanuel Frossard, examiner Prof. Jaco Le Roux, co-examiner
Dr. Beat Frey, co-examiner Dr. Philippe Lemanceau, co-examiner
1
2
Contents
Abstract 2
Glossary 7
General introduction 9
Chapter 1 29
Chapter 2 51
Chapter 3 97
Chapter 4 142
General discussion 171
Acknowledgements 198
Curriculum vitae 201
Appendices 203
3
Per a la Kati, de tot cor
4
Abstract
Rhizobia are bacteria specialized in making atmospheric nitrogen available to legumes, playing a key role in the support of legume growth and establishment in managed and natural ecosystems. The relationship between rhizobial diversity and plant performance is not yet clear.
Many studies show lower rhizobial diversity is more beneficial to plant growth, but at the same time soils and legume roots contain large amounts of rhizobial and other root-associated bacterial diversity. This suggests we need to better understand the ecological factors that drive the diversity and composition of these microbes in space and time.
Understanding the ecological processes driving the diversity of rhizobia and other root- associated bacteria is a prerequisite to potentially manage their beneficial functions to plants.
This thesis explores the biogeography and ecological drivers of root nodule bacterial diversity across temporal and spatial scales in rooibos (Aspalathus linearis), a South African-endemic legume crop. The main goal is to understand which factors determine rhizobial diversity and composition at the plant, field, and regional scales, and in both cultivated and wild rooibos populations.
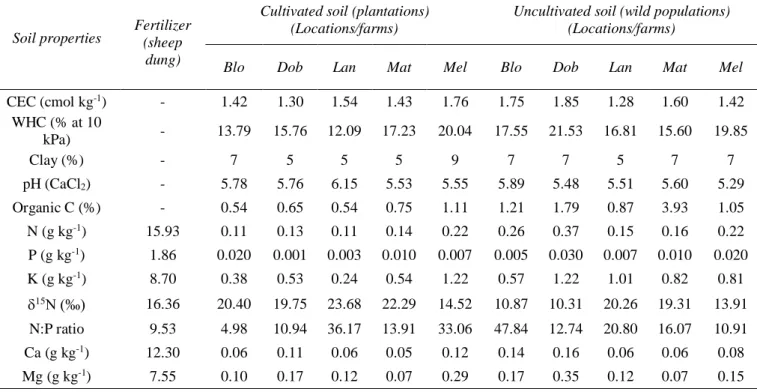
Rooibos is adapted to acidic and sandy soils very poor in mineral nutrients and organic matter, and grows under low rainfall conditions (150-450 mm y-1. Rooibos has multiple adaptations to withstand mineral nutrient limitation, among which rhizobial symbioses play a central role.
Rooibos associates to a diverse range of rhizobial genera, Mesorhizobium being the dominant group, but frequently interacting with Bradyrhizobium, Rhizobium, and Burkholderia. The plant is particularly interesting to study ecological drivers of rhizobial diversity because 1) plantations lie adjacent to wild populations across the landscape; 2) the plant grows in a climatically and edaphically heterogeneous region with high speciation rates; and 3) the crop has been recently domesticated (~70 years), providing a good analog to study rhizobial diversity before and after crop domestication.
This thesis is based on a dual marker approach, in which root nodule bacterial communities of rooibos were characterized using a functional and a taxonomic gene markers. We sequenced a 455 bp-long fragment of the rhizobial gene nodA (encoding an N-acyltransferase important to induce root nodulation), and a 817 bp-long fragment of the taxonomic marker gene gyrB (encoding a topoisomerase common to all bacterial taxa). This combination ensured a majority of the bacterial community of rooibos root nodules could be described.
5
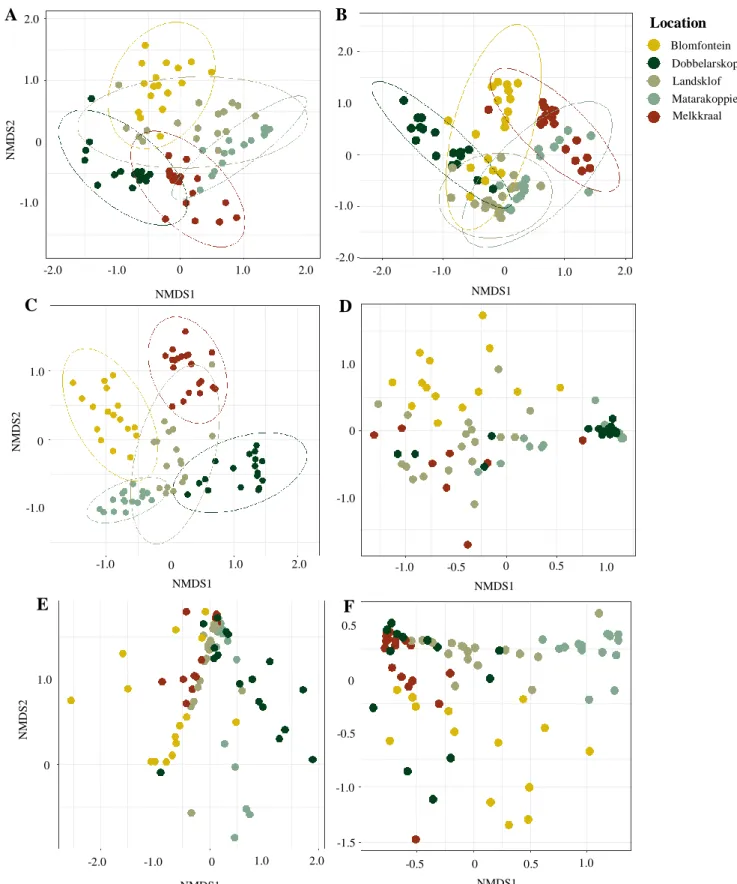
The thesis starts providing a framework to assess how root microbial symbioses differ between cultivated and the ancestral counterparts of legumes. Given the coexistence of cultivated and wild populations, rooibos appears as a good study case upon which the framework could be developed. This framework provided theoretical and experimental basis to assess the ability of different cultivars to associate to, and benefit from, rhizobia from their wild ancestors. This provided a baseline from which a pot experiment growing rooibos on soils from cultivated and uncultivated soils was conceived. The aim of this experiment was to address how geographical and soil abiotic factors drive the diversity of root nodule bacteria in rooibos seedlings. The addition of sheep dung in the experiment revealed the diversity of rhizobia colonizing root nodules was weakly dependant on the soil environment, and strongly related to stochastic assembly processes driven by root biomass and geographic isolation. Rhizobial community composition displayed a strong geographical pattern, whereby different farms contained distinct rhizobial communities, and these became more different with geographic distance.
Instead, the communities from plants grown on cultivated and uncultivated soils had low levels of dissimilarity.
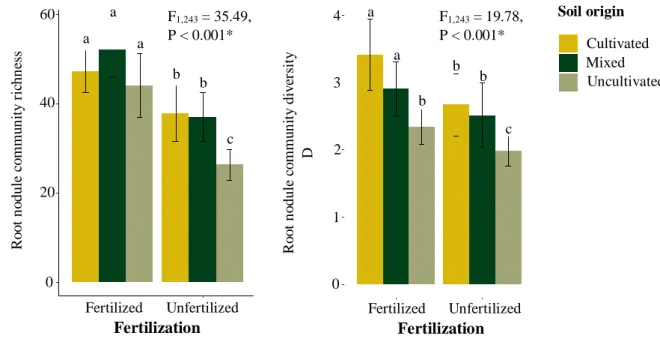
In a field survey, root nodules from cultivated and wild rooibos plants were collected, and their diversity was related to rooibos foliar nutrient concentrations, plant genotype, geographical location, and soil nutrient concentrations. This revealed that rooibos is nodulated by a core of dominant and widespread Mesorhizobium strains, but whose dominance depends on the geographical location more than any other factor. Organic rooibos cultivation maintained high levels of root nodule diversity at the field level, but had a homogenizing effect at the regional scale. There were established weak but significant links between rooibos foliar nutrient concentrations and rhizobial community structure, suggesting different rooibos populations may harbour distinct root nodule communities.
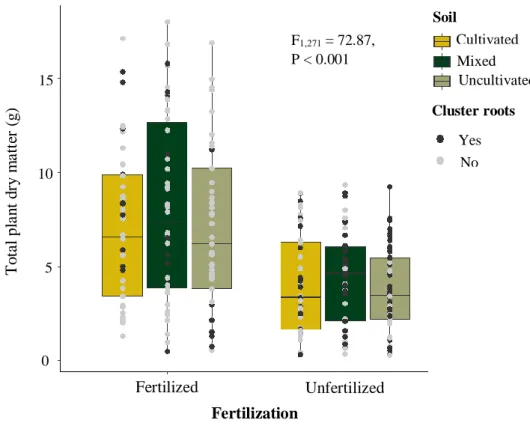
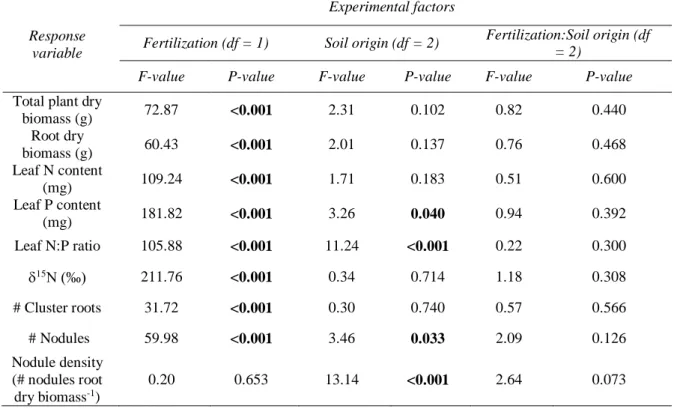
In the pot experiment soils from cultivated and wild rooibos populations were mixed, as a way to increase the available rhizobial pools in the soil. This so-called community coalescence events could introduce rhizobial diversity from wild rooibos populations to arable land after seedling transplantation. This revealed that fertilization, despite not changing the composition of rhizobial communities, promoted the dominance of a larger number of taxa. It had a synergistic effect with soil mixing, whereby a Mesorhizobium OTU that was rare in plants grown on soils from cultivated and wild rooibos populations became dominant only after combining soil mixing and fertilizer addition. Higher soil rhizobial diversity, however, had no influence on any plant response.
6
In summary, through the description of the predominant root nodule bacterial groups, this thesis shows that rooibos root nodule diversity is geographically structured, and weakly affected by the measured environmental factors. Rooibos cultivation appears as a homogenizing force of rhizobial diversity at regional scales, while soil mixing appears as an alternative to associate rooibos seedlings to rhizobia from unmanaged populations locally. Overall, this thesis shows that the ecological drivers of root nodule diversity differ across scales: at the root level, diversity increases probabilistically with root biomass; at the population level, local soil abiotic factors and potentially plant ecotypes define the composition of root nodule communities; and regionally, barriers to dispersal seem to drive divergence between rooibos root nodule communities. Over time, some evidence suggests rhizobial diversity is filtered out as the post- fire succession proceeds in the progress of rooibos from seedling to maturity. Future research should address how agronomic practices in rooibos farming can promote potential benefits of rhizobial symbioses under changing climatic conditions.
7
Zusammenfassung
Rhizobien sind Bakterien welche darauf spezialisiert sind, Hülsenfrüchten Luftstickstoff zur Verfügung zu stellen. Sie spielen eine Schlüsselrolle bei der Unterstützung des Hülsenfrüchtewachstums in der Landwirtschaft und natürlichen Ökosystemen. Trotz der Wichtigkeit von Rhizobien ist der Zusammenhang zwischen Rhizobienvielfalt und Pflanzenleistung noch nicht geklärt. Viele Studien zeigen, dass eine geringe Rhizobialvielfalt für das Pflanzenwachstum vorteilhaft ist, aber gleichzeitig enthalten Wurzelknöllchen und die Rhizosphäre (Bodenumgebung der Wurzeln) von Hülsefrüchten eine grosse Vielfalt an Rhizobien. Dies legt nahe, dass wir die ökologischen Faktoren welche die Vielfalt und Zusammensetzung dieser Mikroben in Raum und Zeit bestimmen, besser verstehen müssen.
Ein Verständnis der ökologischen Prozesse, welche die Vielfalt der Rhizobien und anderer wurzelassoziierter Bakterien vorantreiben, ist eine Voraussetzung für das potenzielle Management ihrer nützlichen Funktionen für Pflanzen. Diese Arbeit untersucht die Biogeographie und die ökologischen Faktoren der bakteriellen Vielfalt der Wurzelknöllchen über zeitliche und räumliche Skalen in Rooibos (Aspalathus linearis), einer südafrikanischen endemischen Hülsenfrucht. Das Hauptziel ist es, Faktoren welche die Vielfalt und Zusammensetzung der Rhizobien auf pflanzlicher, Feld- und regionaler Ebene sowie in kultivierten und wilden Rooibospopulationen zu bestimmen.
Rooibos ist an saure und sandige Böden angepasst, die sehr arm an Mineralstoffen und organischen Stoffen sind, und wächst unter geringen Niederschlagsbedingungen (150-450 mm y-1) in der Cape Floristic Region Südafrikas. Eine wichtige Anpassung von Rooibos an die sehr nährstoffarmen Bedingungen ist die Symbiose mit Rhizobien. Rooibos assoziiert mit einer Vielzahl von Rhizobien, wobei Mesorhizobium die dominante Gruppe ist, aber häufig assoziiert die Pflanze auch mit Bradyrhizobium, Rhizobium oder Burkholderia. Die Pflanze ist besonders interessant für die Untersuchung ökologischer Treiber der Rhizobienvielfalt, da 1) Plantagen an nicht kultivierte Populationen in der gesamten Landschaft angrenzen; 2) die Pflanze in einer klimatisch und edaphisch heterogenen Region mit hoher Artenvielfalt wächst;
und 3) die Kultur erst vor kurzem domestiziert wurden (~70 Jahre). Die Nähe von kultivierten und wilden Pflanzen wie auch die kurze Zeit seit der Domestikation eignen sich um den Einfluss von Domestikation auf die Rhizobienvielfalt anhand von Rooibos zu untersuchen.
8
In dieser Arbeit wurde ein Dual-Marker-Ansatz verwendet: bakterielle Gemeinschaften von Rooibos in Wurzelknöllchen wurden mit einem funktionellen und einem taxonomischen Marker charakterisiert. Wir sequenzierten ein 455 bp langes Fragment des Rhizobiengens nodA (genetische Code für eine N-Acyltransferase, die für die Induktion der Wurzelmodulation wichtig ist) und ein 817 bp langes Fragment des taxonomischen Markergens gyrB (genetische Code für eine Topoisomerase, die allen Bakterientaxa gemeinsam ist). Die Kombination aus diesen Markern stellte sicher, dass ein Großteil der Bakteriengemeinschaft der Rooiboswurzelknollen beschrieben werden konnte.
Es gibt Hinweise darauf, dass die Domestikation und Züchtung von Nutzpflanzen die mikrobiellen Symbiosen der Wurzeln in einigen modernen Nutzpflanzen beeinträchtigt haben.
Die Dissertation bietet einen Rahmen, um zu untersuchen, vie sich microbielle Wurzelsymbiosen zwischen kultivierten Leguminosen und den Wildpflanzenpopulationen unterscheiden. Angesichts der Koexistenz von kultivierten und unkultivierten Populationen ist Rooibos ein gutes Modell, um diese These zu testen. Dies bildete die Grundlage für einen Topfversuch, bei dem Rooibos auf Böden aus kultivierten und nicht kultivierten Böden angebaut wurde. Ziel dieses Experiments war es, zu untersuchen, wie geografische und Management Faktoren die Vielfalt der Wurzelknöllchenbakterien in Rooiboskeimlingen beeinflussen. Die Zugabe von Schafmist im Experiment ergab, dass die Vielfalt der Rhizobien, die Wurzelknollen besiedeln, schwach von der Bodenumgebung abhängig war und stark mit stochastischen Montageprozessen zusammenhängt, die durch Wurzelbiomasse angetrieben werden. Darüber hinaus zeigte die Zusammensetzung der Rhizobiengemeinschaften ein starkes geographisches Muster, wobei verschiedene Farmen unterschiedliche Rhizobiengemeinschaften enthielten. Die Unterschiede zwischen den Farmen vergrösserten sich mit zunehmender geographischer Entfernung.
In einer Felduntersuchung wurden Wurzelknöllchen von kultivierten und wilden Rooibospflanzen gesammelt, und deren Vielfalt mit den Blattnährstoffkonzentrationen des Rooibos, dem Pflanzengenotyp, der geografischen Lage und den Nährstoffkonzentrationen des Bodens korrelliert. Dabei stellte sich heraus, dass Rooibos von einem Kern aus dominanten und weit verbreiteten Mesorhizobium-Stämmen bevölkert wird, dessen Dominanz jedoch mehr als jeder andere Faktor von der geografischen Lage abhängt. Der biologische Rooibosanbau hielt die Vielfalt der Wurzelknöllchen auf Feldebene hoch, hatte aber einen homogenisierenden Effekt auf regionaler Ebene. Es wurden schwache, aber signifikante Verbindungen zwischen den Blattnährstoffkonzentrationen des Rooibos und der Struktur der Rhizobiengemeinschaft
9
festgestellt. Dies deutet darauf hin, dass verschiedene Rooibos-Populationen unterschiedliche Wurzelknöllchengemeinschaften aufweisen können.
In einem weiteren Topfversuch wurden Böden aus kultivierten und wilden Rooibospopulationen gemischt, um die verfügbare Rhizobienvielfalt im Boden zu erhöhen.
Diese so genannten gemeinschaftlichen Koaleszenzereignisse könnten nach der Setzlingstransplantation die Vielfalt der Rhizobien von wilden Rooibospopulationen auf Ackerland übertragen. Die Untersuchung ergab, dass Schafmistdüngung, die Zusammensetzung der Rhizobiengemeinschaften nicht veränderte, wohl aber die Dominanz einer größeren Anzahl von Taxa förderte. Die Düngerbeigabe hatte einen synergistischen Effekt mit der Bodenvermischung, wobei eine Mesorhizobium OTU, die in Pflanzen auf Böden aus kultivierten und wilden Rooibospopulationen selten war, erst nach der Kombination von Bodenvermischung und Düngerzugabe dominant wurde. Die höhere Boden-Rhizobienvielfalt hatte jedoch keinen messbaren Einfluss auf das Pflanzenwachstum.
Zusammenfassend lässt sich sagen, dass Rooibos--Wurzelknöllchenvielfalt geographisch strukturiert ist, und nur schwach abhängig von anderen gemessenen Umweltfaktoren. Der Rooibosanbau wirkt als homogenisierende Kraft der Rhizobienvielfalt auf regionaler Ebene.
Bodenmischung scheint eine pragmatische Lösung zu sein, um Rooiboskeimlingen mit Rhizobien aus wilden Populationen zu impfen. Insgesamt zeigt diese Arbeit, dass die ökologischen Treiber der Wurzelknöllchendiversität in den verschiedenen Skalen unterschiedlich sind: Auf der Wurzelebene nimmt die Vielfalt mit der Wurzelbiomasse zu; auf der Populationsebene definieren lokale abiotische Bodenfaktoren und potenzielle Pflanzenökotypen die Zusammensetzung der Wurzelknöllchengemeinschaften; und regional können Hindernisse für die Verbreitung die Divergenz zwischen den Wurzelknöllchengemeinschaften fördern. Zukünftige Forschungen sollten untersuchen, wie agronomische Praktiken in der Rooibos-Landwirtschaft den potenziellen Nutzen von Rhizobiensymbiosen unter sich ändernden klimatischen Bedingungen fördern können.
10
Glossary
Biological invasion A species acquiring a competitive advantage following the disappearance of natural obstacles to its proliferation. This allows it to spread rapidly and to conquer novel areas in which it becomes a dominant population.
Co-adaptation Process by which two species adapt as a pair or group, which occurs when two or more interacting traits undergo natural selection together.
Community assembly Accumulation of species diversity in a particular habitat over time.
Eco-Evolutionary Degree of familiarity of a species with the biotic environment in
Experience (EEE) which it is found.
Ecological community Set of populations of different species that coexist in space and time.
Ecological drift Process that makes the abundance of particular species fluctuate stochastically over time, which is prevalent when population sizes are small.
Ecological insurance Process by which biological diversity maintains ecosystem functions under perturbation because more diverse assemblages are more likely to contain species that perform particular functions.
Plant ecotype A distinct form of a plant species adapted to a particular habitat.
Environmental filtering Process by which species fail to establish and persist in a novel habitat because the environmental conditions to not support their survival.
Functional redundancy Phenomenon by which different species perform similar or identical functions in a given environment.
Plant genotype Set of alleles that define the genetic makeup of a plant and determine its traits.
Holobiont Discrete biological unit composed by a host and all species living in or around it.
Neutral assembly Process by which the assembly of species in an ecological community is driven by random dispersal, speciation and extinction, and not by the species’ adaptations to the environment.
11
Ecological niche The functional position of a species in its habitat, including the habitat itself, the resources it obtains from it, and its interactions with other species. Theoretically, two different species cannot occupy exactly the same niche in the same habitat at the same time.
Priority effect Impact that a species has on community development due to its early arrival at a particular site. The effect can be facilitative, by favouring the establishment of other species, or inhibitory, by impairing the establishment of other species.
Rhizosphere The region of soil in the vicinity of plant roots in which the chemistry and microbiology is influenced by their growth, respiration, and nutrient exchange.
Symbiosis Interaction between two different organisms living in close physical association, typically to the advantage of both.
Symbiotic promiscuity Capacity of an organism to establish symbioses with multiple species.
Trait-based assembly Process by which the assembly of species in an ecological community is driven by the adaptations of species to the environment.
12
General introduction
13
Microbial diversity and the functioning of the legume-rhizobium symbiosis
The legume-rhizobium symbiosis is a paradigmatic and debated case of high microbial community functionality at low diversity levels, and is at the core of the present thesis.
Rhizobia belong to the α- and β-Proteobacteria and are facultative endosymbionts specialized in the fixation and transfer of atmospheric nitrogen (N2) to host plants and promotion of plant growth (Denison and Kiers, 2011). These bacteria propagate endosymbiotically in structures called root nodules, which protect them from the exterior environment, and ensure the exchange of plant-available N forms and carbohydrates. The nodules maintain low O2 levels critical for the proper functioning of the nitrogenase, the enzyme responsible for the reduction of atmospheric N2 into ammonia (NH3) (Denison and Kiers, 2011). After root nodule decay, rhizobial propagules are released to the rhizosphere, where they survive as saprotrophs (i.e.
decomposing organic matter with extracellular enzymes; Moawad et al., 1984).
Plants and rhizobia have co-evolved mechanisms to interact and ensure symbiotic functionality (West et al., 2002). As in any mutualistic system involving multiple taxa, both rhizobial strains and the plant incur in energy investments to maintain the mutualism. This means that rhizobia not investing into N2 fixation would have an advantage over N2-fixing ones, thus leading to the breakdown of mutualism (Denison, 2000). This has the consequence that with more different rhizobia in the nodules the likelihood that non-cooperation appears increases, as diversity also gives more chances for non-cooperating rhizobia to be found in the nodules. To avoid this, plants have evolved mechanisms to sanction ineffective rhizobia while rewarding most cooperative ones (Kiers, 2003). This is done by reducing investment into nodules with low effectiveness and preferentially allocating photosynthetates into the best performing ones. This consequently decreases root nodule diversity (which is distinct from rhizobial diversity as it includes potentially non-symbiotic bacteria present in the nodules) (Denison and Kiers, 2011).
From the plant’s perspective, ensuring the optimal functioning of the best performing rhizobial strains is the most effective way to preserve symbiotic benefits, and therefore the potential functional redundancy and complementarity from higher rhizobial diversity levels becomes a priori dispensable (Oono et al., 2009). If this is true, then why do we observe high levels of rhizobial diversity in the root nodules of some legumes?
Only a few studies have addressed this question, which has been defined as a paradox, both empirically and theoretically (Heath and Stinchcombe, 2014; Pahua et al., 2018). By comparing the growth of acacias inoculated with single versus mixed rhizobial strains, Barrett
14
et al., (2015) showed that mixed inoculations decreased plant productivity. The authors argued that antagonism between strains, for example through the production of bacteriocins (Triplett and Sadowsky, 1992), or the hormonal inhibition of nodulation by non-cooperative strains (Hogg et al., 2002), could explain such observations. Similar results were obtained in Medicago truncatula, where mixed rhizobium infections impaired plant growth compared to the worst single strain inoculation (Heath and Tiffin, 2007). The apparent disconnect between rhizobial diversity and mutualistic benefits may require a change in the temporal and spatial scales at which it is studied (Bever et al., 2009). For example, it has been demonstrated that the coexistence of different host plants with different preference for rhizobial partners can favour the maintenance of diverse rhizobial communities (Pahua et al., 2018). While at the root nodule scale rhizobial strain-strain interactions can decrease symbiotic benefits, the reality is that over longer time scales environmental conditions and the plant’s physiological status change. Thus, not only in space but also over time, different rhizobial strains have different fitness advantages, which can maintain the transfer of N to the host under changing environmental conditions (Heath and Stinchcombe, 2014; Bever, 2015). This fact has steered a few studies that have concluded that, on the long run, rhizobial diversity may have a reason to be (Siler and Friesen, 2017).
Diversity and functions of rhizobial symbioses in agricultural plant production
Biologically fixed nitrogen is a renewable nitrogen source, which is central to the benefits agricultural plant production draws from rhizobial symbioses (Zahran, 1999). The amount of N2 symbiotically fixed in arable land annually is in the range of 10-500 kg N ha-1 (Peoples et al., 2009), while the total amount is estimated to be 20-22 Tg N (Herridge et al., 2008). The rhizobial symbiosis can contribute up to 70% of N inputs from fertilizers annually, making cropping systems involving legumes less reliant on N derived from fertilizers (Lasaletta et al., 2014). While the use of rhizobial inoculants has proved beneficial, it has so far been undermined by the strong biotic resistance of local microbial communities present in the soil (Mathu et al., 2012; Burghardt, 2019). Moreover, the establishment capacity and effectiveness of particular rhizobial strains has made rhizobial inoculation reliant on very low rhizobial diversity. At the same time, tillage, biomass removal, soil homogenisation, pest control and monocropping of genetically homogenous crop varieties is known to change soil microbial community composition in arable land (Hartmann et al., 2015). Together, these observations
15
suggest new approaches are needed towards managing lasting and effective root microbial symbioses in plant production (Toju et al., 2018).
The lack of knowledge on how and why rhizobial communities display particular diversity levels hampers our ability to control and promote their benefits in plant production (Busby et al., 2017). While complex microbial communities with particular functions can be manipulated in laboratory conditions (Bell, 2019), it is not yet clear how these would be sustained in the field. For root nodule communities, a way forward can be to ensure the co-adaptability of host plant and microbes to the soil and climatic conditions (Heath and Tiffin, 2007; Vuong et al., 2017). This degree of matching has been instrumental in explaining legume range expansions and invasions in partnership with their site-adapted or compatible rhizobial symbionts (Le Roux et al., 2017). For example, non-native acacia trees co-dispersing with their native (i.e.
compatible) rhizobia have overall higher success in establishing in new areas (Richardson et al., 2000; Rodríguez-Echeverría et al., 2012). Provided they tolerate the prevailing environmental conditions, the extra N input these plants receive from their co-adapted symbionts gives them a head-start in the colonization of new areas. Notions learned from invasion ecology can thus be of great use in efforts to foster rhizobial mutualisms and their diversity in cropping systems.
Eco-evolutionary mechanisms to promote the long-term stability of rhizobial symbioses with modern crops
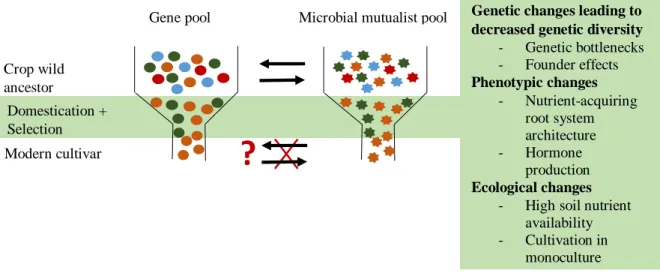
In a pioneering study, Kiers et al., (2007) demonstrated that legume crop domestication and breeding may have compromised the ability of modern cultivars to benefit from rhizobial communities. Plants under breeding are known to incur in domestication syndromes, whereby the subsequent narrowing of their genetic pools is associated with the loss of multiple adaptive traits found in the ancestral populations (Doebley et al., 2006; Gaut et al., 2018). Narrower genetic pools derive from processes common to the domestication and breeding process such as genetic bottlenecks and founder effects (Gaut et al., 2018). These processes lead to the loss of genetic variation due to severe reductions in the crop population size, and to the cropping of only small subsets of the original population respectively. In parallel, breeding under elevated nutrient inputs may have decreased the reliance of crops on the additional nutrients afforded by their symbionts, consequently allowing for the spread of legumes unable to control rhizobial infection (Kiers et al., 2007; Weese et al., 2015; see Sawers et al., 2018 and Martín-Robles et al., 2018 for the analogous case in arbuscular mycorrhizal fungi) (Fig. 1).
16
Eco-evolutionary experience (EEE) is a term used to explain the success of biological invasions that can bridge mechanisms of invasion ecology and rhizobial symbioses in plant production.
The term encapsulates the importance of prolonged contact between organisms (i.e. biotic interactions) as a way for one organism to establish in new ecological communities (Saul and Jeschke, 2013; Saul et al., 2015). Modern cultivars face challenges to establish in new areas in which adaptations to the local symbionts or the soil and climatic conditions are missing, suggesting the EEE concept can help. From the symbiosis point of view, modern cultivars face two major challenges: 1) successive artificial selection rounds can have eroded the genes enabling symbiotic interactions (Sawers et al., 2017; von Wettberg et al., 2018); and 2) expansion of the cropping areas causing decreases in rhizobial diversity to which cultivars are adapted to. Therefore, accounting for EEE can help improve crops’ adaptability to the prevailing environmental conditions by maintaining the symbiotic interactions the plant is naturally involved in. A comprehensive review on the use of the EEE and other invasion ecology concepts to promote stable rhizobial symbioses in modern crops is provided in Chapter 1, which has been published recently (Ramoneda et al., 2019).
An emerging conclusion from this is that we need to better understand how rhizobial diversity is maintained during the assembly in the root nodules of legume crops both in plantations and,
Figure 1 Proposed link between modern crop gene pools and their microbial mutualist associations.
Increasing evidence suggests genetic bottlenecks at the domestication, selection and breeding stages may explain why particular root symbioses are being impaired in modern crops.
Genetic changes leading to decreased genetic diversity - Genetic bottlenecks - Founder effects Phenotypic changes
- Nutrient-acquiring root system architecture - Hormone
production Ecological changes
- High soil nutrient availability - Cultivation in
monoculture Consequences for crop-mutualist functioning:
- Reduced crop responsiveness to mutualistic interactions.
- Reduced selectivity or ability for mutualist recruitment by the crop.
- Impaired acquisition of additional nutrients from the mutualism under changing conditions.
- Negative feedbacks between decreased soil microbial diversity and mutualist reruitment driven by high nutrient availability.
Crop wild ancestor
Modern cultivar
Gene pool Microbial mutualist pool
Domestication + Selection
?
17
when possible, populations of wild ancestors of the crop (Pérez-Jaramillo et al., 2018). This is of particular importance in legumes adapted to changing and/or nutrient-poor conditions, in which rhizobial diversity is expected to provide an ecological insurance in the face of perturbation (Yachi and Loreau, 1999). The comparison of rhizobial associations in modern cultivars and crop wild relatives is a further step required in order to address whether there are beneficial functions from rhizobial diversity that can be obtained from the wild ancestors of modern crops (Bulgarelli et al., 2015; Pérez-Jaramillo et al., 2018). Several inoculation studies have addressed the influence of different microbial symbiont inocula on the performance of different plant genotypes (Lehmann et al., 2012 for a meta-analysis; Pérez-Jaramillo et al., 2017; Leff et al., 2017; Martín-Robles et al., 2018). However, these have never incorporated long-term effects of symbiont diversity on plant fitness, and only a small proportion of modern crops contain extant wild populations to compare to. This means finding a suitable study system may not be trivial, and a major objective of the present thesis is to describe a plant-rhizobium system that allows for such ancestor-modern cultivar comparison.
Study system: Rooibos (Aspalathus linearis) in the Cape Floristic Region, South Africa
Rooibos (Aspalathus linearis) is a legume of the monophyletic Subtribe Crotalariae (Fabaceae;
Gepts et al., 2005). Aspalathus is the largest endemic plant genus of South Africa with 279 species (Dahlgren 1988; Cupido 2007; Malgas et al., 2010), and represents one of several yet unresolved phylogenetic lineages of the genistoid legumes, with Crotalaria spp., lupines and peanut being its closest relatives. Small shrubs in the genus Aspalathus radiated in the rugged landscape of the Cederberg Mountain Range (Western Cape, ZA), assumingly driven by habitat patchiness and fire-driven dynamics, leading to rapid speciation and slow extinction rates (Cowling et al., 2009). In particular, rooibos shows high ecotypic variation in the wild (Malgas et al., 2010; Hawkins et al., 2011), which reflects different strategies to cope with soil nutrient and water availability. This is driven by nutrient flushes after natural bushfires occurring at intervals of about ten years (Lötter et al., 2014), as rooibos is a post-fire microsuccessional pioneer (Stock and Lewis, 1986; Allsopp and Stock, 1992).
Rooibos is cultivated within the semi-arid (150-450 mm y-1) scrublands of the South African Cape Floristic Region (CFR; Lötter and Le Maitre, 2014; Fig. 2), and commercialized to the
18 international markets as
rooibos tea (a caffein- and tannin-free beverage). The nutrient-leached, acidic, and sandy soils are among the lowest in mineral (micro)nutrients and organic matter worldwide, the latter leading to poor water retention in cultivation. When not
directly sown in the top 5cm of soil, rooibos seedlings are grown in nurseries for 6-8 months prior to transplantation (Wynberg, 2017; pers. comm. Noel Oettlé, Environmental Monitoring Group, Nieuwoudtville, ZA). Up to two thirds of the aboveground biomass are harvested annually for 4-7 years, when commonly an oat intercrop is planted for 1-3 years for soil recovery (see Appendix 1 for information on rooibos farming history and practices).
Noteworthy, wild rooibos populations lie adjacent to the plantations. Cultivated rooibos represents an apparently homogeneous gene pool as a single reseeding ecotype, which is reported to have hybridized with conspecific uncultivated ecotypes. This has been favoured by pollination by bees and wasps, and post-fire seed dispersal by ants (Malgas et al., 2010) (Fig.
3).
Rooibos has the ability to associate with both α- (mostly from the genera Bradyrhizobium, Mesorhizobium, and Rhizobium; Le Roux et al., 2017b) and β-rhizobia (the genus Burkholderia; Hassen et al., 2012; Stepkowski et al., 2018). Comparative studies including close relatives of rooibos provide evidence for biogeographic co-structuring of rhizobial and legume occurrence in the CFR (Lemaire et al., 2015; Le Roux et al., 2017b). In both cultivated and wild rooibos populations, the genus Bradyrhizobium is dominant in the soil, whereas rooibos root nodules are dominated by Mesorhizobium taxa (Le Roux et al., 2017b). Other genera that have been isolated from rooibos root nodules are Agrobacterium, Methylobacterium, and Sinorhizobium (Gamper and Ramoneda 2017, unpublished). Rooibos also establishes interactions with arbuscular mycorhizal fungi (AMF) (Allsopp and Stock, 1992), which assist in phosphorus and water acquisition, in concert with modified root
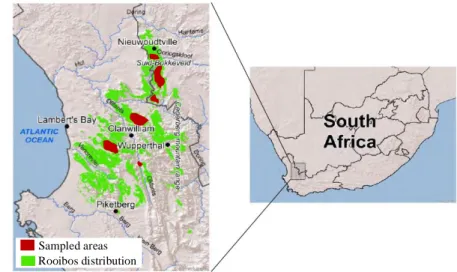
Rooibos distribution Sampled areas
Figure 2 Map of the rooibos distribution range and areas sampled in the present study (Western and Northern Cape, South Africa).
19
appendages called cluster roots (Maseko and Dakora, 2013; Gamper and Ramoneda 2017, unpublished, Appendix 2).
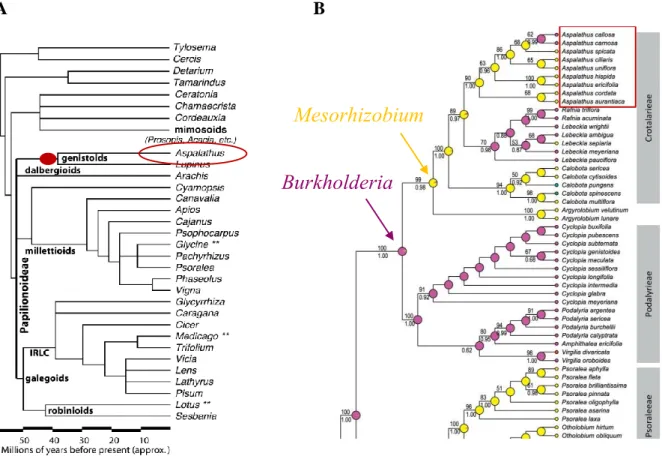
The establishment and subsequent geographic isolation of distinct legume populations in the CFR has been ascribed to the facilitative role of the genus Mesorhizobium in the adaptation to a wide range of soil pH levels (Stepkowski et al., 2018). Indeed, a recent phylogenetic study pointed at symbiont switching between Burkholderia (low pH specialist) and Mesorhizobium (pH range generalist), as a potentially important eco-evolutionary mechanism in the radiation of Aspalathus species in South Africa (Dludlu et al., 2018, Fig. 4). Such co-diversification could have several implications for the rooibos domestication process: (1) active ecotypic differentiation driven by heterogeneous soil conditions in the landscape would be linked to different rhizobial assemblages potentially beneficial in plantation; (2) soils naturally similar to those in plantation (e.g. lowest in organic matter, located in areas with the lowest rainfall), might have more beneficial rooibos genotype x rhizobial strain interactions that could be
Figure 3 Wild habitats and cultivation systems of rooibos [Aspalathus linearis (Burm.f.) R.Dahlgr., Crotalariae]. The species is endemic to and cultivated in the megadiverse Cape Floristic Region of South Africa.
(A) Wild rooibos habitat: Sandstone Fynbos soils dominated by rush-like restios (Restionaceae) with a scattered occurrence of taller proteas (Proteaceae) (Suid Bokkeveld, Northern Cape, South Africa). (B) Extensive cultivation of rooibos under reduced weed control, allowing the invasion of wild grasses (Graminaceae), (Skimmelberg, Western Cape, South Africa). (C) Intensive cultivation of rooibos plants that were raised in nurseries under irrigation and fertilization. (D) Organic rooibos farming landscape with plantations surrounded by strips of semi-natural fynbos vegetation.
A B
C D
20
deliberately targeted; and (3) domestication of a single rooibos genotype might start a new plant-rhizobial co-evolutionary trajectory of adaptation to the agronomic management
conditions (Bell and Tylianakis, 2016), which could trigger the loss of beneficial functional traits (e.g. decreased molecular signalling ability for recruitment of other beneficial rhizobia when abiotic conditions change).
In the edaphically and climatically heterogeneous landscape of the CFR, local plant populations are exposed to distinct environmental conditions at a regional scale (Cowling et al., 2009), known to also affect the survival of rhizobial communities (Sprent et al., 2017). At the local population level, rooibos hosts distinct rhizobial communities due to local edaphic and climatic filters (Le Roux et al., 2017b), and likely due to neighbouring vegetation (Pahua et al., 2018).
At the root level, rhizobial community assembly may depend solely on the selection, matching and filtering by the plant (Denison and Kiers, 2011). In symbiotically promiscuous plants like rooibos, filtering seems to be weak as the strong growth-limiting conditions in its habitat do not allow the plant to be selective (West et al., 2002). Together, the combination of barriers to
Burkholderia Mesorhizobium
A B
Figure 4 Evolutionary history of the genus Aspalathus in relation to the dominant rhizobial symbionts of rooibos. (A) Phylogenetic position of the genus Aspalathus within the Family Papilionidae, showing an approximated radiation about 40 million years ago (modified from Cannon et al., 2009). (B) Phylogenetic relationship of the genus Aspalathus with other members of the Subtribe Crotalariae and reconstructed dominant rhizobial genera (Burkholderia in pink, Mesorhizobium in yellow; modified from Dludlu et al., 2018).
21
dispersal and ecological legacies regionally, environmental filters locally, and a lack of selection at the root community level, make rooibos a prominent case for studying the multiple dominant drivers of rhizobial diversity across different spatial scales.
Objectives, research questions and structure of this thesis
The main goal of this thesis is to understand the ecological factors that structure rhizobial communities and their diversity at the plant, field, and landscape scales in rooibos. State-of- the-art next generation DNA sequencing of a taxonomic (gyrB) and a functional (nodA) gene markers in the studied rhizobial populations was used to describe these communities. As an important crop with extant wild populations, the description of rooibos rhizobial communities in this work addresses the following fundamental research questions:
1) Which are the dominant ecological drivers (i.e. soil abiotic factors, rooibos genotype, geographical distance and isolation, rooibos plant traits) that determine the root nodule diversity and composition of rooibos in space and time?
2) How does rooibos cultivation impact the diversity of root nodule communities at the plant, agricultural field, and regional scales?
3) Are there any symbiotic benefits associated to rhizobial diversity that can be promoted by manipulating soil nutrient contents (i.e. fertilization) and increasing the available rhizobial diversity in the soil?
The thesis transitions from a conceptual and experimental framework developed in Chapter 1, whose insights serve as a basis for the experimental and observational approaches used in the subsequent chapters. The main rationale is that rooibos, as a recently domesticated crop with extant wild populations adjacent to plantations, and characterized by a history of plant colonization of new habitats, is an ideal system to study rhizobial biogeography and the potential loss of rhizobial diversity with cropping. Short summaries of the aims of the different chapters are provided hereunder:
Chapter 1 reviews evidence for root microbial symbioses contributing to the adaptability and establishment of plants into new environments. The objective is to translate insights learned from the plant invasion literature to the study of symbioses in modern crops. The framework of eco-evolutionary experience (EEE) is proposed as a basis on which cross-inoculation experiments involving crops and their wild relatives should be conducted. The outcomes of such experiments can inform whether modern crops maintain the ability to benefit from their
22
ancestral rhizobial symbionts, which could be important under changing environmental conditions.
Chapter 2 is based on a pot experiment in which rooibos seedlings were grown on soils from cultivated and wild rooibos populations from distinct geographical locations. The objective was to reveal the ways in which simple agronomic measures such as fertilization with sheep dung and soil mixing affect the diversity and composition of root nodule communities in rooibos. Additionally, the effects of the geographical origin of the soil were tested as an important contributor to root nodule diversity across rooibos populations.
Chapter 3 is based on a field survey in which root nodule communities from conventional and organically cultivated and wild rooibos populations are compared across the plant’s distributional range. The objective was to describe the diversity and composition of the dominant root nodule communities of rooibos, and to disentangle the relative effects of dominant drivers (i.e. geographical distance, population type, rooibos genotypic identity and soil nutrient concentrations) that may shape community diversity and structure. To this end, a community prediction approach, never used in rhizobial research before, was used to define the core rhizobial assemblages in rooibos root nodules.
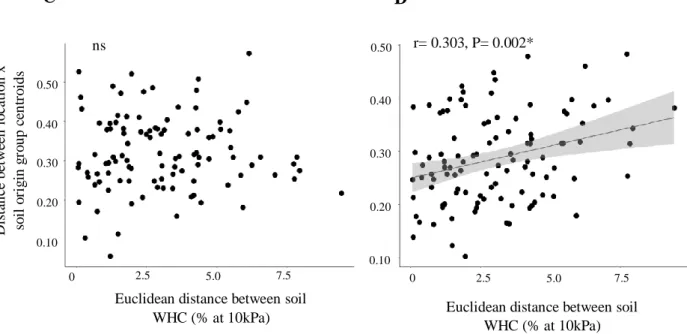
Chapter 4 focuses on the effects of increased soil nutrient content on the dominance of rhizobial taxa introduced through soil mixing. The objective was to address if the addition of sheep dung in combination with the mixing of cultivated and uncultivated soils influenced rhizobial dominance in the same way across farms. This work revealed that sheep dung input gives chances to a wider range of rhizobia to dominate nodule colonization, while soil mixing allows rare taxa to become dominant. This implies sheep dung addition and soil mixing can act synergistically to favour new rhizobial interactions in rooibos seedlings, and can be a way to (re-)introduce rhizobial taxa from wild populations of the crop.
23
References
Allsopp, N., & Stock, W. D. (1992). Density dependent interactions between VA mycorrhizal fungi and even-aged seedlings of two perennial Fabaceae species. Oecologia, 91(2), 281–
287.
Bell, T., & Tylianakis, J. M. (2016). Microbes in the anthropocene: Spillover of agriculturally selected bacteria and their impact on natural ecosystems. Proceedings of the Royal Society B: Biological Sciences, 283(1844).
Bell, T. (2019). Next-generation experiments linking community structure and ecosystem functioning. Environmental Microbiology Reports, 11(1), 20–22.
Bever, J. D., Richardson, S. C., Lawrence, B. M., Holmes, J., & Watson, M. (2009).
Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecology Letters, 12(1), 13–21.
Bever, J. D. (2015). Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytologist, 205(4), 1503–1514.
Bulgarelli, D., Garrido-Oter, R., Münch, P. C., Weiman, A., Droge, J., Pan, Y., McHardy, A.
C., & Schulze-Lefert, P. (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host and Microbe, 17(3), 392–403.
Burghardt, L. T. (2019). Evolving together, evolving apart: measuring the fitness of rhizobial bacteria in and out of symbiosis with leguminous plants. New Phytologist, 1–7.
Busby, P. E., Soman, C., Wagner, M. R., Friesen, M. L., Kremer, J., Bennett, A., Morsy, M., Eisen, J. A., Leach, J. E., & Dangl, J. L. (2017). Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biology, 15(3), 1–14.
Cannon, S. B., May, G. D., & Jackson, S. A. (2009). Three sequenced legume genomes and many crop species: Rich opportunities for translational genomics. Plant Physiology, 151(3), 970–977.
24
Cowling, R. M., Procheş, Ş., & Partridge, T. C. (2009). Explaining the uniqueness of the Cape flora: Incorporating geomorphic evolution as a factor for explaining its diversification.
Molecular Phylogenetics and Evolution, 51(1), 64–74.
Cupido, C. N. (2007). Notes on African plants: Fabaceae. Aspalathus theresae, a new species from Western Cape, South Africa. Bothalia, 37, 34-38.
Dahlgren, R. (1988). Crotalarieae (Aspalathus). Flora of Southern Africa, 16, 84-90.
Denison, R. F. (2000). Legume sanctions and the evolution of symbiotic cooperation by rhizobia. American Naturalist, 156(6), 567–576.
Denison, R. F., & Kiers, E. T. (2011). Life histories of symbiotic rhizobia and mycorrhizal fungi. Current Biology, 21(18), 775–785.
Dludlu, M. N., Chimphango, S. B. M., Walker, G., Stirton, C. H., & Muasya, A. M. (2018).
Horizontal gene transfer among rhizobia of the Core Cape Subregion of southern Africa.
South African Journal of Botany, 118, 342–352.
Doebley JF, Gaut BS, S. B. (2006). The molecular genetics of crop domestication. Cell, 127(7), 1309–1321.
Gaut, B. S., Seymour, D. K., Liu, Q., & Zhou, Y. (2018). Demography and its effects on genomic variation in crop domestication. Nature Plants, 4(8), 512–520.
Gepts, P., Beavis, W. D., Brummer, E. C., Shoemaker, R. C., Stalker, H. T., Weeden, N. F., &
Young, N. D. (2005). Legumes as a model plant family: genomics for food and feed.
Plant Physiol, 137, 1228–1235.
Godsoe, W., Jankowski, J., Holt, R. D., & Gravel, D. (2017). Integrating Biogeography with Contemporary Niche Theory. Trends in Ecology and Evolution, 32(7), 488–499.
Graham, E. B., Knelman, J. E., Schindlbacher, A., Siciliano, S., Breulmann, M., Yannarell, A.,
… Nemergut, D. R. (2016). Microbes as engines of ecosystem function: When does community structure enhance predictions of ecosystem processes? Frontiers in Microbiology, 7, 1–10.
25
Hajjar, R., & Hodgkin, T. (2007). The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica, 156(1–2), 1–13.
Hartmann, M., Frey, B., Mayer, J., Mäder, P., & Widmer, F. (2015). Distinct soil microbial diversity under long-term organic and conventional farming. ISME Journal, 9(5), 1177–
1194.
Hassen, A. I., Bopape, F. L., Habig, J., & Lamprecht, S. C. (2012). Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-Proteobacteria and β-Proteobacteria. Biology and Fertility of Soils, 48(3), 295–303.
Hawkins, H. J., Malgas, R., & Biénabe, E. (2011). Ecotypes of wild rooibos (Aspalathus linearis (Burm. F) Dahlg., Fabaceae) are ecologically distinct. South African Journal of Botany, 77(2), 360–370.
Heath, K. D., & Tiffin, P. (2007). Context dependence in the coevolution of plant and rhizobial mutualists. Proceedings of the Royal Society B: Biological Sciences, 274(1620), 1905–
1912.
Heath, K. D., & Stinchcombe, J. R. (2014). Explaining mutualism variation: A new evolutionary paradox? Evolution, 68(2), 309–317.
Herridge, D.F., Peoples, M.B. & Boddey, R.M. (2008). Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil, 311(1-2), 1-18.
Hogg, B., Davies, A. E., Wilson, K. E., Bisseling, T., & Downie, J. A. (2002). Competitive nodulation blocking of cv. Afghanistan pea is related to high levels of nodulation factors made by some strains of Rhizobium leguminosarum bv. viciae. Molecular Plant-Microbe Interactions, 15(1), 60–68.
Kiers, E. T., Rousseau, R. A., West, S. A., & Denison, R. F. (2003). Host sanctions and the legume–\nrhizobium mutualism. Nature, 425, 78–81.
Kiers, E. T., Hutton, M. G., & Denison, R. F. (2007). Human selection and the relaxation of legume defences against ineffective rhizobia. Proceedings of the Royal Society B:
Biological Sciences, 274(1629), 3119–3126.
26
Knelman, J. E., & Nemergut, D. R. (2014). Changes in community assembly may shift the relationship between biodiversity and ecosystem function. Frontiers in Microbiology, 5, 1–4.
Lassaletta, L., Billen, G., Grizzetti, B., Anglade, J., & Garnier, J. (2014). 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environmental Research Letters, 9(10).
Leff, J. W., Lynch, R. C., Kane, N. C. & Fierer, N. (2017). Plant domestication and the assembly of bacterial and fungal communities associated with strains of the common sunflower, Helianthus annuus. New Phytologist 214(1), 412–423.
Lehmann, A., Barto, E. K., Powell, J. R., & Rillig, M. C. (2012). Mycorrhizal responsiveness trends in annual crop plants and their wild relatives-a meta-analysis on studies from 1981 to 2010. Plant and Soil 355(1–2), 231–250.
Leibold, M. A., Holyoak, M., Mouquet, N., Amarasekare, P., Chase, J. M., Hoopes, M. F., … Gonzalez, A. (2004). The metacommunity concept: A framework for multi-scale community ecology. Ecology Letters, 7(7), 601–613.
Lemaire, B., Dlodlo, O., Chimphango, S., Stirton, C., Schrire, B., Boatwright, J. S., … Muasya, A. M. (2015). Symbiotic diversity, specificity and distribution of rhizobia in native legumes of the Core Cape Subregion (South Africa). FEMS Microbiology Ecology, 91(2), 1–42.
Lemanceau, P., Maron, P. A., Mazurier, S., Mougel, C., Pivato, B., Plassart, P., … Wipf, D.
(2014). Understanding and managing soil biodiversity: a major challenge in agroecology.
Agronomy for Sustainable Development, 35(1), 67–81.
Le Roux, J. J., Hui, C., Keet, J. H., & Ellis, A. G. (2017a). Co-introduction vs ecological fitting as pathways to the establishment of effective mutualisms during biological invasions.
New Phytologist, 215(4), 1354–1360.
Le Roux, J. J., Keet, J. H., Mutiti, B., & Ellis, A. G. (2017b). Cultivation may not dramatically alter rhizobial community diversity or structure associated with rooibos tea (Aspalathus linearis Burm.f.) in South Africa. South African Journal of Botany, 110, 87–96.
27
Lötter, D., & le Maitre, D. (2014). Modelling the distribution of Aspalathus linearis (Rooibos tea): Implications of climate change for livelihoods dependent on both cultivation and harvesting from the wild. Ecology and Evolution, 4(8), 1209–1221.
Lotter, D., Valentine, A. J., Archer Van Garderen, E., & Tadross, M. (2014). Physiological responses of a fynbos legume, Aspalathus linearis to drought stress. South African Journal of Botany, 94, 218–223.
Malgas, R. R., Potts, A. J., Oettlé, N. M., Koelle, B., Todd, S. W., Verboom, G. A., & Hoffman, M. T. (2010). Distribution, quantitative morphological variation and preliminary molecular analysis of different growth forms of wild rooibos (Aspalathus linearis) in the northern Cederberg and on the Bokkeveld Plateau. South African Journal of Botany, 76(1), 72–81.
Martín-Robles, N., Lehmann, A., Seco, E., Aroca, R., Rillig, M. C., & Milla, R. (2018). Impacts of domestication on the arbuscular mycorrhizal symbiosis of 27 crop species. New Phytologist, 218(1), 322–334.
Maseko, S. T., & Dakora, F. D. (2013). Plant Enzymes, Root Exudates, Cluster Roots and Mycorrhizal Symbiosis are the Drivers of P Nutrition in Native Legumes Growing in P Deficient Soil of the Cape Fynbos in South Africa. Journal of Agricultural Science and Technology, 3, 331–340.
Mathu, S., Herrmann, L., Pypers, P., Matiru, V., Mwirichia, R., & Lesueur, D. (2012) Potential of indigenous bradyrhizobia versus commercial inoculants to improve cowpea (Vigna unguiculata L. walp.) and green gram (Vigna radiata L. wilczek.) yields in Kenya, Soil Science and Plant Nutrition, 58(6), 750-763.
Mendes, R., Garbeva, P., & Raaijmakers, J. M. (2013). The rhizosphere microbiome:
Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiology Reviews, 37(5), 634–663.
Moawad, H. A., Ellis, W. R., & Schmidt, E. L. (1984). Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Applied and Environmental Microbiology, 47(4), 607–612.
28
Mueller, U. G., & Sachs, J. L. (2015). Engineering Microbiomes to Improve Plant and Animal Health. Trends in Microbiology, 23(10), 606–617.
Oono, R., Denison, R. F., & Kiers, E. T. (2009). Controlling the reproductive fate of rhizobia:
How universal are legume sanctions? New Phytologist, 183(4), 967–979.
Pahua, V. J., Stokes, P. J. N., Hollowell, A. C., Regus, J. U., Gano-Cohen, K. A., Wendlandt, C. E., … Sachs, J. L. (2018). Fitness variation among host species and the paradox of ineffective rhizobia. Journal of Evolutionary Biology, 31(4), 599–610.
Peoples, M. B., Brockwell, J., Herridge, D. F., Rochester, I. J., Alves, B. J. R., Urquiaga, S., ...
& Sampet, C. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis, 48(1), 1-17.
Pérez-Jaramillo, J. E., Carrión, V. J., Bosse, M., Ferrão, L. F. V., De Hollander, M., Garcia, A.
A. F., … Raaijmakers, J. M. (2017). Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME Journal 11(10), 2244–2257.
Pérez-Jaramillo, J. E., Carrión, V. J., de Hollander, M., & Raaijmakers, J. M. (2018). The wild side of plant microbiomes. Microbiome, 6(1), 4–9.
Pholchan, M. K., de Baptista, J. C., Davenport, R. J., Sloan, W. T., & Curtis, T. P. (2013).
Microbial community assembly, theory and rare functions. Frontiers in Microbiology, 4, 1–9.
Plassart, P., Prévost-Bouré, N. C., Uroz, S., Dequiedt, S., Stone, D., Creamer, R., … Lemanceau, P. (2019). Soil parameters, land use, and geographical distance drive soil bacterial communities along a European transect. Scientific Reports, 9(1), 1–17.
Ramoneda, J., Le Roux, J., Frossard, E., Bester, C., Oettlé, N., Frey, B., & Gamper, H. (2019).
Insights from invasion ecology: Can consideration of eco-evolutionary experience promote benefits from root mutualisms in plant production? AoB Plants, DOI: 10.1093/aobpla/plz060/5572512.
29
Richardson, D.M., Allsopp, N., D’Antonio, C., Milton, S.J. & Rejmánek, M. (2000) Plant invasions – the role of mutualism. Biological Reviews, 75, 65–93.
Rodríguez-Echeverría, S., Fajardo, S., Ruiz-Díez, B., & Fernández-Pascual, M. (2012).
Differential effectiveness of novel and old legume-rhizobia mutualisms: Implications for invasion by exotic legumes. Oecologia, 170(1), 253–261.
Saul, W.-C., Jeschke, J., & Heger, T. (2013). The role of eco-evolutionary experience in invasion success. NeoBiota, 17, 57–74.
Saul, W. C., & Jeschke, J. M. (2015). Eco-evolutionary experience in novel species interactions. Ecology Letters, 18(3), 236–245.
Sawers, R. J. H., Svane, S. F., Quan, C., Grønlund, M., Wozniak, B., Gebreselassie, M. N., … Paszkowski, U. (2017). Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytologist, 214(2), 632–643.
Sawers, R. J., Ramírez‐Flores, M. R., Olalde‐Portugal, V., & Paszkowski, U. (2018). The impact of domestication and crop improvement on arbuscular mycorrhizal symbiosis in cereals: insights from genetics and genomics. New Phytologist, 220(4), 1135-1140.
Siler, E., & Friesen, M. L. (2017). Widespread Negative Frequency-Dependent Selection Maintains Diversity in the Legume-Rhizobia Symbiosis Balancing nodulation may explain the paradox of rhizobium diversity. BioRxiv, 1–15.
https://doi.org/10.1101/153866.
Socolar, J. B., Gilroy, J. J., Kunin, W. E., & Edwards, D. P. (2016). How Should Beta-Diversity Inform Biodiversity Conservation? Trends in Ecology and Evolution, 31(1), 67–80.
Sprent, J. I., Ardley, J., & James, E. K. (2017). Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytologist, 215(1), 40–56.
Stępkowski, T., Banasiewicz, J., Granada, C. E., Andrews, M., & Passaglia, L. M. P. (2018).
Phylogeny and phylogeography of rhizobial symbionts nodulating legumes of the tribe genisteae. Genes, 9(3).
30
Stock, W. D., & Lewis, O. a M. (1986). Soil nitrogen and the role of fire as a mineralizing agent in a South African coastal fynbos ecosystem. Journal of Ecology, 74(2), 317–328.
Tanksley, S. D., & McCouch, S. R. (1997). Seed banks and molecular maps: Unlocking genetic potential from the wild. Science, 277(5329), 1063–1066.
Teshima, H., Hirao, K., Toriyama, M., & Kanzaki, S. (1999). Fabrication and mechanical properties of silicon nitride ceramics with unidirectionally oriented rodlike grains.
Journal of the Ceramic Society of Japan, 107(12), 1216–1220.
Toju, H., Peay, K. G., Yamamichi, M., Narisawa, K., Hiruma, K., Naito, K., ... & Yoshida, K.
(2018). Core microbiomes for sustainable agroecosystems. Nature Plants, 4(5), 247.
Triplett, E. W., & Sadowsky, M. J. (1992). Genetics of competition for nodulation of legumes. Annual Review of Microbiology, 46(1), 399-422.
Von Wettberg, E. J. B., Chang, P. L., Başdemir, F., Carrasquila-Garcia, N., Korbu, L. B., Moenga, S. M., … & Singh, V. (2018). Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nature Communications 9, 649.
Vuong, H. B., Thrall, P. H., & Barrett, L. G. (2017). Host species and environmental variation can influence rhizobial community composition. Journal of Ecology, 105(2), 540–548.
Weese, D. J., Heath, K. D., Dentinger, B. T. M., & Lau, J. A. (2015). Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution, 69(3), 631–642.
West, S. A., Kiers, E. T., Simms, E. L., & Denison, R. F. (2002). Sanctions and mutualism stability: Why do rhizobia fix nitrogen? Proceedings of the Royal Society B: Biological Sciences, 269(1492), 685–694.
Wynberg, R. (2017). Making sense of access and benefit sharing in the rooibos industry:
Towards a holistic, just and sustainable framing. South African Journal of Botany, 110, 39–51.
Yachi, S., & Loreau, M. (1999). Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences, 96, 1463–1468.
31
Zahran, H. H. (1999). Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and Molecular Biology Reviews, 63(4), 968–989.
Zamir, D. (2001). Improving plant breeding with exotic genetic libraries. Nature Reviews Genetics, 2(12), 983–989.
32
Chapter 1
Insights from invasion ecology: Can consideration of eco-evolutionary experience promote benefits from root mutualisms in plant production?
Josep Ramoneda1, Johannes Le Roux2, Emmanuel Frossard1, Cecilia Bester3, Noel Oettlé4, Beat Frey5, Hannes Andres Gamper6
1Group of Plant Nutrition, Department of Environmental Systems Science, ETH Zurich, Zurich, Switzerland.
2Department of Biological Sciences, Macquarie University, Sydney, Australia.
3South African Agricultural Research Council (ARC-Infruitec), Stellenbosch, South Africa.
4Environmental Monitoring Group (EMG), Nieuwoudtville, South Africa.
5Rhizosphere Processes Group, Swiss Federal Research Institute WSL, Birmensdorf, Switzerland.
6Institute of Life Sciences, Scuola Superiore Sant’Anna, Piazza Martiri della Libertà 33, 56127 Pisa, Italy
Published as: Ramoneda, J., Le Roux, J., Frossard, E., Bester, C., Oettlé, N., Frey, B., &
Gamper, H. (2019). Insights from invasion ecology: Can consideration of eco-evolutionary experience promote benefits from root mutualisms in plant production? AoB Plants, DOI: 10.1093/aobpla/plz060/5572512.
33
Abstract
Mutualistic plant-microbial functioning relies on co-adapted symbiotic partners as well as conducive environmental conditions. Choosing particular plant genotypes for domestication and subsequent cultivar selection can narrow the gene pools of crop plants to a degree that they are no longer able to benefit from microbial mutualists. Elevated mineral nutrient levels in cultivated soils also reduce the dependence of crops on nutritional support by mutualists such as mycorrhizal fungi and rhizobia, the nutritionally relevant microbial symbionts of crops.
Thus, current ways of crop production are predestined to compromise the propagation and function of microbial symbionts, limiting their long-term benefits for plant yield stability. The influence of mutualists on non-native plant establishment and spread, i.e. biological invasions, provide an unexplored analogue to contemporary crop production that accounts for mutualistic services from rhizobia and mycorrhizae. The historical exposure of organisms to biotic interactions over evolutionary timescales, or so-called eco-evolutionary experience (EEE), has been used to explain the success of such invasions. In this paper, we stress that consideration of the EEE concept can shed light on how to overcome the loss of microbial mutualist functions following crop domestication and breeding. We propose specific experimental approaches to utilize the wild ancestors of crops to determine whether crop domestication compromised the benefits derived from root microbial symbioses or not. This can predict the potential success of mutualistic symbiosis manipulation in modern crops and the maintenance of effective microbial mutualisms over the long term.
34
Introduction
Rapid climate change and the need to utilize resources more efficiently call for crops that are able to cope with perturbation and stress to support stable yields. Root microbial mutualists such as arbuscular mycorrhizal fungi (AMF, phylum Glomeromycota; Tedersoo et al., 2019) and rhizobia (α- and β-Proteobacteria; Masson-Boivin and Sachs 2018) have the ability to benefit and increase crops’ access to additional nutrients and reduce abiotic and biotic stress.
As a consequence, they improve the ability of crops to cope with increasingly unpredictable and changing abiotic and biotic conditions (Denison and Kiers 2011; Lau and Lennon 2012;
Hurst 2017). For example, legumes have co-evolved with their microbial symbionts, particularly with rhizobia, which partly explains their ability to thrive under and adapt to both novel and rapidly changing environments (Porter et al., 2011; Sprent et al., 2017; Masson- Bovin and Sachs 2018). Likewise, some promiscuous legumes have taken advantage of interactions with unfamiliar rhizobia during range expansion to survive novel edaphic and climatic conditions (Simonsen et al., 2017). This is facilitated by the additional access to N, P, water and micronutrients afforded by rhizobia and AMF (Sprent et al., 2017). Microbial symbionts may also adapt to newly encountered abiotic conditions (e.g. soil pH; Dludlu et al., 2017) which, in turn, influence the performance and benefits received by host plants (Bennett and Klironomos 2018).
The symbiotic functioning of root-associated microbes can be drastically altered depending on host plant identity and local environmental conditions (Kiers et al., 2003; Martín-Robles et al., 2018; von Wettberg et al., 2018). This is important to bear in mind as crop domestication, breeding and cultivation are changing the genetic and ecological conditions under which plants are grown, which can compromise the benefits crops derive from microbial symbioses (Hetrick et al., 1992; Kiers et al., 2003; Sawers et al., 2018). For instance, cultivation under high mineral nutrient availability is expected to reduce allocation of photosynthetates to symbiotic microbes (Kiers et al., 2003, 2007). The easy access to abundant soil resources for crops grown under high soil fertility can cause decreased dependency on mutualisms. These relaxed conditions may, in turn, lead to the accumulation of deleterious mutations in symbiosis-related genes in the plant genome. This can lead to compromised abilities of crops to recruit and reward microbial mutualists over the long run (Hetrick et al., 1992; Doebley et al., 2006; Kiers et al., 2007; Leff et al., 2017; von Wettberg et al., 2018). Additionally, selection for reduced
![Figure 3 Wild habitats and cultivation systems of rooibos [Aspalathus linearis (Burm.f.) R.Dahlgr., Crotalariae]](https://thumb-eu.123doks.com/thumbv2/1library_info/5483873.1684828/21.892.113.783.181.606/figure-habitats-cultivation-systems-rooibos-aspalathus-linearis-crotalariae.webp)